Researchers from Osaka University have made a groundbreaking discovery in the field of autophagy, a crucial cellular process that holds the key to understanding aging and various diseases. Their findings shed light on the intricate mechanisms behind the initiation of autophagy, paving the way for new therapeutic approaches.
The Vital Role of Autophagy
Autophagy is a fundamental cellular process that allows cells to recycle and eliminate damaged or unwanted components. This self-degradation process is not only essential for maintaining cellular homeostasis but also plays a crucial role in various aspects of health and disease.
During autophagy, intracellular structures and molecules are enclosed within a membrane-bound compartment called an autophagosome, which is then delivered to the lysosomes for degradation. This remarkable mechanism helps cells adapt to changing environmental conditions, provide a source of nutrients, and clear out potentially harmful substances, making it a crucial player in aging, cancer, and neurodegenerative disorders.
Understanding the precise mechanisms that trigger and regulate autophagy has been a longstanding challenge in the scientific community. The new findings from the Osaka University research team shed light on this crucial process, opening up new avenues for therapeutic interventions and the promotion of healthy aging.
Unveiling the Autophagy Trigger
The research team, led by Osaka University, has made a groundbreaking discovery in the field of autophagy. They have identified a novel mechanism that is crucial for the initiation of this cellular self-degradation process.
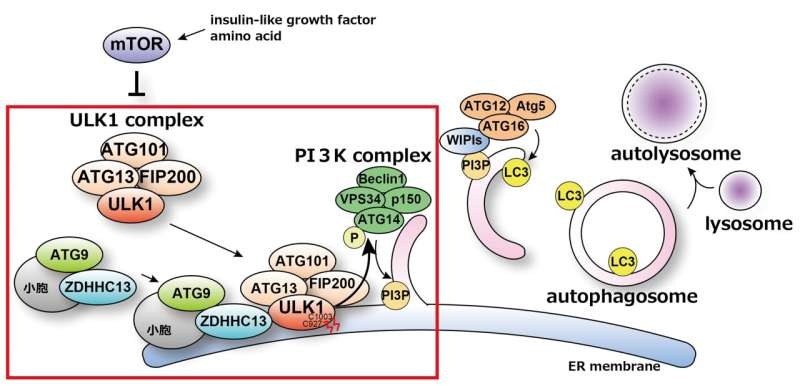
Previously, the researchers had revealed that the formation of autophagosomes, the membrane-bound structures that engulf cellular components, takes place on the endoplasmic reticulum (ER) membrane, in close proximity to mitochondria. They also discovered that the PI3K complex, an autophagy-related protein, is essential for this formation process.
Now, the team has uncovered the underlying mechanism that triggers the relocation of the ULK1 complex, a key player in autophagy, from the cytoplasm to the ER membrane, where autophagosomes are formed. The researchers found that the palmitoylation of ULK1, catalyzed by the enzyme ZDHHC13, is the crucial step that initiates this critical translocation.
This palmitoylation not only localizes the ULK1 complex to the autophagosome formation sites but also regulates the activity of the PI3K complex, another essential component in the autophagy process. By understanding these intricate molecular mechanisms, the researchers have shed light on the long-standing mystery of how autophagy is triggered and regulated within cells.
Conclusion
The groundbreaking discovery by the Osaka University research team has significantly advanced our understanding of the mechanisms behind the initiation of autophagy. By unveiling the role of ULK1 palmitoylation and its impact on the PI3K complex, the researchers have provided a crucial piece of the puzzle in deciphering the complex signaling pathways that govern this fundamental cellular process.
This knowledge has far-reaching implications, as autophagy is not only essential for cellular homeostasis but also plays a pivotal role in various disease states, including cancer, neurodegenerative disorders, and aging. The insights gained from this study can pave the way for the development of targeted therapies and novel interventions aimed at modulating autophagy, potentially leading to breakthroughs in the treatment and prevention of a wide range of health conditions.
