A team of researchers has developed a new process that enables unprecedented conversion efficiency of ammonia to hydrogen, and is now poised for testing at a pilot-plant scale.
Using the Power of Ammonia
Hydrogen gas is being looked at as a thriving basis of uncontaminated and capable grounds results for a manageably resonance. But hydrogen is for the most part bound and the difficult to extract.
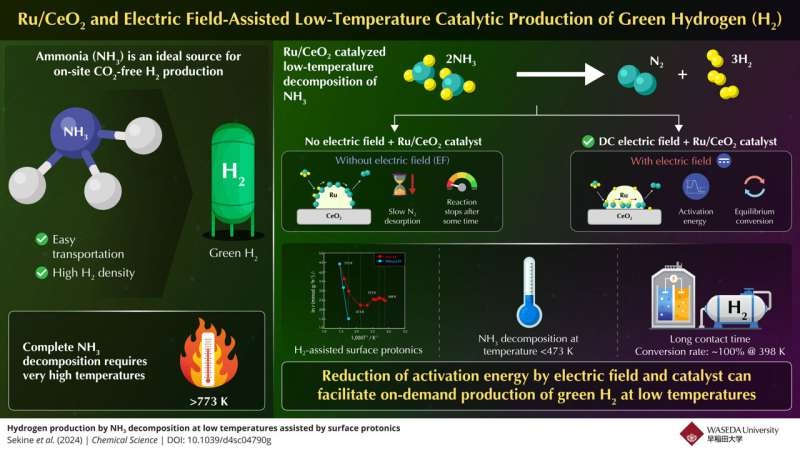
Ammonia has emerged as one of the key contenders among all hydrogen carriers. Because ammonia has a high hydrogen content, can be easily liquefied, and is more widely available than regular hydrogen, it offers the potential for a step change in how we produce and use hydrogen. Ammonia decomposition, by contrast, remains prohibitively high temperature for practical on-demand hydrogen production using the traditional thermal route.
One team of researchers from Waseda University and Yanmar Holdings has developed a new method to convert ammonia into hydrogen at lower temperatures using electric fields, breaking the conventional process barrier.
The Power of Electric Fields
Professor Yasushi Sekine, who led the research team of Yukino Ofuchi, Sae Doi and Kenta Mitarai explained: ‘Here we demonstrate a highly active Ru/CeO2 catalyst easily prepared in an experimental apparatus with an applied electric field.
Their exploration, published in Chemical Science, unveils that the electric field-assisted catalytic reaction significantly enhances proton conduction on the surface of the catalyst, and lowers the activation energy needed for ammonia conversion as well as reaction temperatures.
The team studied and found that the rate-limiting step in traditional thermal catalytic systems was the desorption of nitrogen under lower temperatures, while the dissociation of N–H bonds at higher temperatures. The electric field eliminated these challenges and it was possible to realize efficient ammonia decomposition at temperatures as low as 473 K.
The researchers discovered during their experiments that a 100% conversion rate was attainable at merely 398 K, which exceeds the equilibrium conversion rate for this temperature as well after leaving the ammonia feed in contact with the catalyst long enough. The excellent performance could be rationalized in terms of the electric field-enhanced surface protonics (e.h., surface proton hopping) on the catalyst, where this lowering of activation energy might take place.
Conclusion
Research by a team at Waseda University and Yanmar Holdings has the goal of dramatically changing how we produce and use hydrogen. By creating an electric field-assisted low-temperature ammonia-to-hydrogen conversion process, they found a novel way to produce green hydrogen on-demand. This advanced method boosts the level of hydrogen generated and even more importantly opens up a new path towards an energy future characterized by sustainability, whereby clean alternatives to fossil fuels are readily available. This research is the first step toward achieving the ultimate purpose of hydrogen becoming a clean, efficient and appropriate energy sources for hydrogen vehicles.
