An unprecedented discovery in microbiology has revealed that single-celled organisms known as archaea contain a vastly larger collection of ribosomal RNA proteins than was previously believed. While that points to when key events in the history of genetic material management may have taken place, “it also provides new directions for interpretation for DNA data and a better understanding of living cells,” said Zaremba-Niedzwiedzka.
Exploring the cryptic world of microbial histones
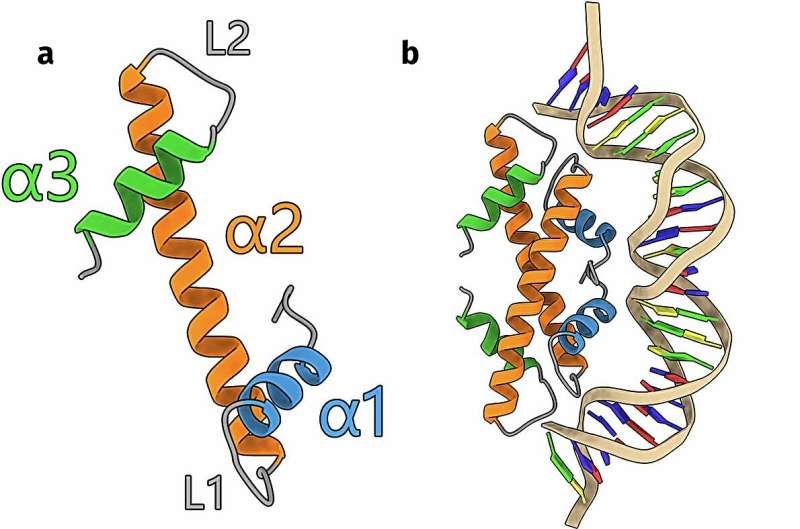
For a long time, it was thought that the intricate organization of DNA that is carried out by histones (proteins) was a feature only found in more complex life forms But these assumptions have been called into question by recent findings from Leiden Ph.D. Samuel Schwab and proved by D candidate and Corporate.
the research by these authors is suggesting that histones are universal and even single-cell organisms including bacteria and archaea have their own versions of histone proteins. Key to understanding how they work are proteins that condense and structure the DNA inside these minuscule organisms. But researchers were truly amazed when they dug deeper into the diversity of these microbial histones.
Applying state-of-the-art AI technology, the team recognised 17 clusters with different histone compositions supporting unique structures and functions. Many were new finds, although some were already known. The very depth of diversity within these smallest of organisms was unheard-of at the time, and it created an entire new area from which to study genetic material control that we know today.
Such is the critical nature of histone in cellular structures
As a way to put this discovery into context, it is seemed intuitive to stress the fundamental position of histones in the building blocks of DNA.
DNA – the genetic toolkit of life is a very long and complicated chain of molecules. In reality, it is so big that in science terms theoretically does not fit into one cell. This is the role of histones Specifically, they serve as a molecular spools that package the DNA into dense complexes of chromatin called nucleosomes. This beautiful setup not only allows DNA to be compact inside the cell; it also controls gene expression and other crucial cellular functions.
Until now, it was thought that these DNA-sequestering histones were unique to complex multicellular organisms. But the research by Schwab and his team has turned this notion on its head, showing that even the simplest life forms have developed sophisticated mechanisms to regulate their genetic raw material. This finding has deep implications for our comprehension of the origin of life and core cellular processes.
Conclusion
This work is both among the first molecular approaches ever to capture a rape genome during Germination for Transformation and gene expression in true Marine-B)|ological systems (B)]Coral|Hydra), as well as an entirely new paradigm for how single cell biology forms its genes. This realization that bacteria and archaea possess an unprecedented diversity in the types of histones, not thought to even be present earlier; represents a dramatic level of rewiring from which knowledge about how life has evolved or worked within cells could spring. I look forward to many more discoveries as the researchers continue to unpack the roles and behaviors of these new histone variants, underlining just how complex and adaptable our microbial world is.
