Scientists from Xi’an Jiaotong University have developed highly efficient and long-lasting oxygen evolution reaction (OER) catalysts. The researchers have discovered a new process for making this material that paves the way for more efficient water splitting and improved renewable energy storage.
Unleashing bimetallic synergies
Abstract: The oxygen evolution reaction (OER) is an essential half-reaction in water splitting, which is an important technology for high-capacity energy storage and sustainable production. Nevertheless, this process is typically limited by slow reaction kinetics that demand a high input of energy.
Precious metal catalysts which serve as traditional OER catalysts (Ru and Ir) exhibit high activity; however, their scarcity and cost energy the exploration of low cost alternatives based on non-precious metals. The resultant NiFe (oxy)hydroxide (NiFeOOH) has been considered as a promising candidate due to its high activity, low cost and durability.
Molybdenum (Mo) is one of the few elements that has a high valence to be incorporated into NiFeOOH material, and Xiangjiu Guan at Xi’an Jiaotong University led research team found it out. It was believed that such innovative design would enable to tailoring the electronic structure of the catalyst and its electrical conductivity, eliminating overpotential for OER and increasing overall stability in actual operating conditions.
Unlocking Catalytic Potential
Through a combination of empirical experiments and theoretical simulations, the researchers studied the complex interplay between Mo, Ni and Fe in the doped catalyst.
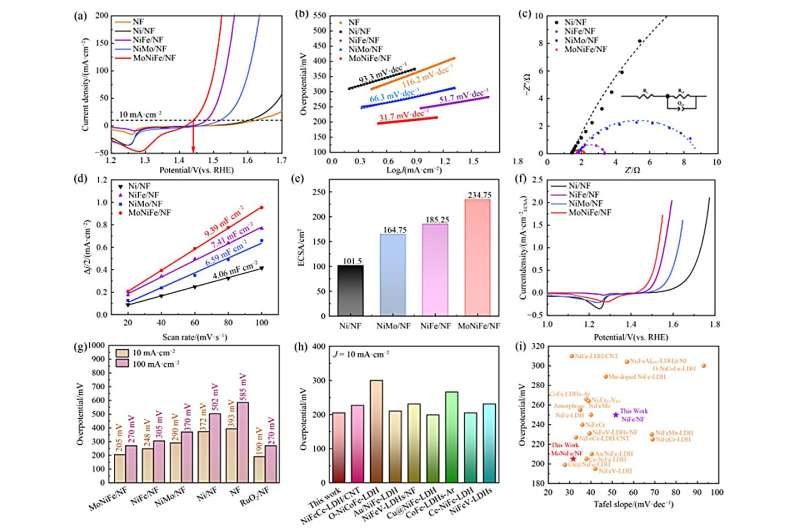
What they found was nothing short of stunning. When Mo is doped, overpotential at 10 mA/cm2 decreased dramatically to 205 mV with Tafel slope of 31.7 mV/dec and the NiFeOOH catalyst became an efficient OER electrocatalyst compared to undoped NiFeOOH [11]. In addition, the catalyst showed stable operation for up to 170 h, indicating a robust performance.
This performance is thanks to the nature of the bimetallic contact pair at play. The d-band center of the bimetallic active sites was shifted to a higher level due to enhanced valence states of Ni and Fe through Mo-doping, which facilitated C─O bond activation. This change is a premise for the conversion to active γ-NiFeOOH phase, which could be beneficial for excellent catalytic activity of the catalyst.
Theoretical studies by density functional theory (DFT) computations corroborated these conclusions and elucidate the most active OER pathways in addition to the role of Mo in promoting catalysis.
Conclusion
Conclusions The pioneering study conducted by Xiangjiu Guan and his team at Xi’an Jiaotong University has been proposing a hopeful prototype of constructing the efficient OER electrocatalysts based on non-precious metals. Therefore, the Mo-doped NiFeOOH catalyst represents a promising bifunctional water-electrolysis catalyst for use in large-scale applications alongside other renewable energy technologies, due to its low overpotential, fast reaction kinetics and long-term stability. Not only does this work provide a mechanistic understanding of bimetallic synergies in catalyst design, our broader strategy holds the promise to inspire further innovations in electrocatalysis — with widespread implications for enabling the transition towards renewable energiesustainability.
