Osaka, Japan — Researchers at Osaka University report a breakthrough discovery in supramolecular chemistry to develop strategies for the precise and controlled synthesis of functionalized materials by self-assembly. They hope the research could lead to new strategies for designing responsive ‘smart materials’ and contribute to riddles ranging from how different shapes emerge in biology, to a fundamental grasp of crystal packing in nature.
Unlocking the Secrets of Self-Assembly
Imagine a world where complex molecules could spontaneously merge to form intricate structures, much like IKEA furniture pieces assembling themselves. This is the realm of supramolecular chemistry, a field of study dedicated to building large molecules from smaller building blocks.
The researchers from Osaka University have taken a significant step forward in this domain, unravelling how the strategic addition of specific chemicals can promote the self-assembly of spherical microparticles. These microparticles, made of a super absorbent polymer called poly(sodium acrylate), were functionalized with two distinct chemical groups: β-cyclodextrin (βCD) and adamantane (Ad) residues.
The key to their success lies in the introduction of a critical threshold concentration of the additive 1-adamantanamine hydrochloride (AdNH3Cl). This additive, inspired by the intricate interactions observed in biological proteins, helps to create a delicate balance of attractions and repulsions between the various chemical components, ultimately guiding the self-assembly process.
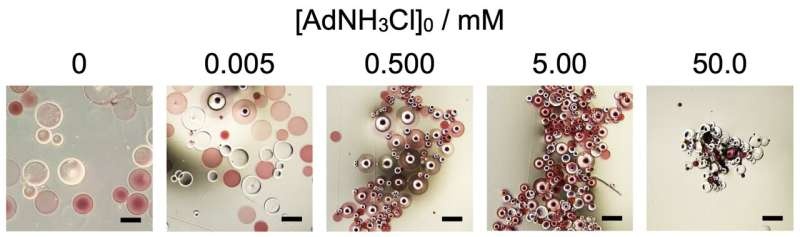
Controlling the Macroscopic Shape
However, the researchers haven’t stopped enabling the self-assembly of spherical microparticles. They further find that the macroscopic shape of these assemblies can be modulated based on the concentration of AdNH3Cl additive, with higher concentrations yielding more spherical and lower concentrations producing elongated assemblies.
This allows us to consider that external stimuli, i.e. heat and force, can be used to change the geometry of these supramolecular aggregates. This has profound implications for packaging and aid to the development of ‘smart materials’ that can respond to stimulus as in nature.
This research transcends the field of materials science. The latter is another point exciting the researchers, who think the plasticity seen in their study might inform more generally about how living forms end up taking different shapes. Need I remind you that, in the words of lead author Akihito Hashidzume, ‘all living organisms are just collections of supramolecular polymers with sophisticated functions.’
Conclusion
This work has been accomplished through the pioneering scientific research of the Osaka University labs into the complex, beautiful world of supramolecular chemistry. They have shown that the self-assembly of spherical microparticles and surprisingly even the macroscopic shape of these assemblies can be controlled by utilizing merely strategic additives. Not only does this research have the potential to completely transform the way we design responsive ‘smart materials’_, but it could also help us understand how we see such interesting shapes in nature. The exploration of the frontiers of supramolecular chemistry is as exciting and unpredictable today as ever, with a wide range of areas available to nurture efforts in innovation and fundamental discoveries.
