Researchers from TU Delft and the Max Planck Institute have developed a groundbreaking class of ‘mechanical’ nanopores made from DNA that can open and close on demand, offering unprecedented control for biomedical applications. These innovative DNA origami nanopores can adjust their diameter, allowing for the selective transport of macromolecules, including potential drug therapies. This landmark achievement paves the way for more advanced dynamic nanodevices that could revolutionize the field of controlled drug delivery and molecular diagnostics.
Obviating the shortcomings related to traditional nanopores
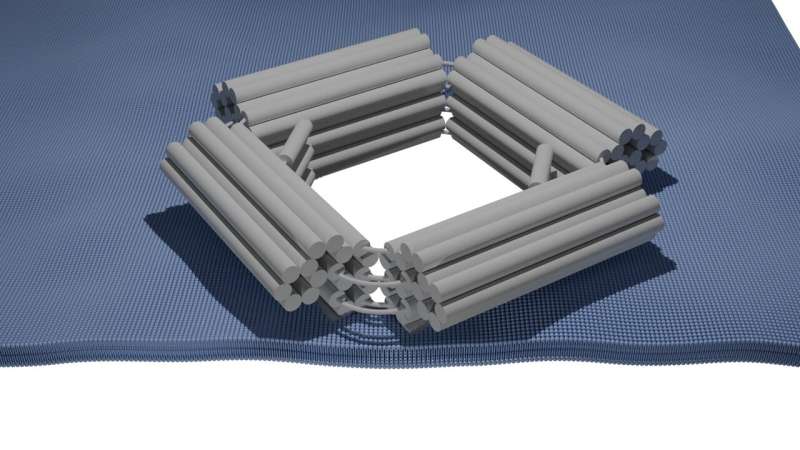
DNA origami nanopores have been extensively applied in biophysics and biotechnology for studying the shapes and compositions of proteins at the single-molecule level. Yet, the pores were too small to deliver large macromolecules including therapeutic drugs. It also is less than efficient at targeted drug delivery, because the pores remain open all the time.
The broader pore with a 30 nanometer opening, created by the researchers at TU Delft and Max Planck Institute provides these capabilities by removing some of the existing challenges. This increase in size is enormously beneficial because it permits the transport of larger biomolecules, a level critical for improved drug delivery systems.
Rediscovering the Potential of Programmable DNA Nanostructures
What makes this all possible is that DNA, as a construction material simply has unique properties. The complementary base pairs are held together by hydrogen bonds, and they utilize these bonds to fold and design 2D or 3D shapes with DNA origami.
This process gave them what seemed like a remarkable amount of control over the constriction of the nanopores itself, leading to not only open and closed states, but easily controllable on-demand. To achieve this, the team introduced flexible single-stranded DNA molecules inside the pore structure. This creates a stiffer double-stranded DNA molecule by helping it open the pore and allow larger biomolecules to pass. On the other hand, the addition of single-stranded DNA molecules that are complementary to the outer DNA molecules makes the pore close; this is a reversible and selective gating mechanism.
Stoichiometry-Dependent Binding and Release from Lipoplexes With Relevance to Targeted and Size-Selective Drug Delivery
Opening and closing the nanopores is not the only advance possible through the researchers’ work. Furthermore, these new MechanoPores can also change their diameter as a result unique size selectivity for molecule transport. This with the possibility to not only separate based on size, but also molecular composition.
The scientists claim that this is the only nanopore able to reversibly achieve three different diameters, which offers a unique means of controlling the flow of biological macromolecules. This feature is of great potential interest in controlled drug delivery, where the transport of therapeutic agents across stable channels can be selective.
The research team will look to improve the nanopore so that proteins can be discriminated based on their molecular contents if they are similar in size, leading to even more targeted and selective drug delivery applications. This makes the prospects of revolutionary biomedical applications, ranging from personalized medicine to superior molecular diagnostics, practically palpable.
