Researchers have discovered that a tiny snippet of RNA, known as SrcF (Segatella RNA colonization factor), plays a vital role in the proliferation and spread of the widespread but understudied bacterium, Segatella copri, within the complex intestinal microbiome. This finding sheds light on the intricate communication and balance that exist among the various microbial species that call our gut home. Microbiome and intestinal bacteria are crucial for human health, and understanding their dynamics could lead to new strategies for maintaining a healthy gut ecosystem.
The Intricate Dance of Gut Microbes
The human intestinal microbiome is a complex and dynamic ecosystem, hosting thousands of different bacterial species that coexist in a delicate balance. These microbes occupy diverse niches and communicate with one another, creating a harmonious co-existence that is crucial for maintaining gut health.
However, certain bacteria, like the ubiquitous Segatella copri, can become more prevalent in specific disease states. Researchers at the Helmholtz Center for Infection Research (HZI) have been studying this bacterium, aiming to understand how it adapts to its environment and the signals it responds to. Their findings, published in the journal Cell Host & Microbe, offer fascinating insights into the role of a small RNA molecule in Segatella copri’s colonization of the gut.
Unraveling the Secrets of Segatella copri
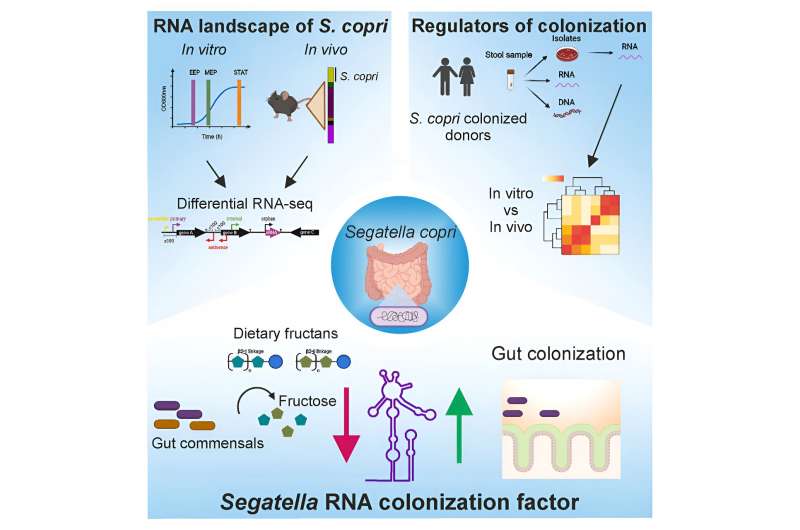
Segatella copri is a widespread bacterium, particularly prevalent in developing and emerging countries, yet it has received relatively little research attention. What is known is that it specializes in the breakdown of dietary fiber, but its precise role in human health remains unclear.
The HZI researchers, led by Prof. Till Strowig, were able to successfully cultivate Segatella copri in the laboratory, a challenging feat. By studying the bacterium’s transcriptome, the researchers discovered a small RNA snippet, dubbed SrcF (Segatella RNA colonization factor), that plays a crucial role in Segatella copri’s ability to multiply and spread within the gut.
The team found that the production of SrcF is triggered by the presence of certain complex carbohydrates, which Segatella copri uses as an energy source. Interestingly, a high concentration of fructans, a type of polysaccharide made up primarily of fructose, was found to suppress SrcF activity, suggesting that the bacterium’s colonization strategies are influenced by the availability of specific nutrients.
The Intricate Balance of the Gut Microbiome
The researchers also discovered that the composition of the gut microbiome itself plays a role in whether Segatella copri activates the SrcF signaling pathway. This highlights the delicate balance that exists among the various microbial species within the gut, as they compete for resources and communicate with one another.
According to Prof. Strowig, the breakdown of large amounts of fructans appears to influence the communication between different intestinal bacteria, ultimately affecting the colonization of Segatella copri. This finding suggests that the gut microbiome’s composition and the availability of specific nutrients can significantly impact the prevalence of certain bacterial species.
The researchers are excited to continue exploring these insights, as a deeper understanding of the gut microbiome’s dynamics could pave the way for new strategies to maintain a healthy and balanced intestinal ecosystem, potentially benefiting human health.
