Researchers have made a significant breakthrough in understanding the precise molecular mechanism of Hsp70 chaperones, which are crucial for protein quality control in cells. Using a cutting-edge nanopore single-molecule technique, they have provided direct evidence for the Entropic Pulling mechanism, shedding light on how these chaperones generate the force needed to manipulate the structure of their client proteins. This research establishes nanopore approaches as a powerful tool for exploring the molecular mechanisms of protein action. Protein folding and chaperone proteins are essential for maintaining cellular function and preventing diseases.
Unraveling the Molecular Secrets of Hsp70 Chaperones
Proteins are the building blocks of life, controlling most of the body’s functions. When they malfunction, it can have severe consequences, such as neurodegenerative diseases or cancer. To maintain protein quality, cells have mechanisms in place, and Hsp70 chaperones play a crucial role in this control system.
Despite their importance, the precise molecular mechanism of Hsp70 chaperones has remained elusive for decades. Researchers at the University of Geneva (UNIGE), in collaboration with the Swiss Federal Institute of Technology Lausanne (EPFL), have now made a significant breakthrough using a cutting-edge nanopore single-molecule technique. They have provided clear evidence for the Entropic Pulling mechanism, which had been proposed but lacked direct experimental confirmation.
Hsp70 Chaperones: Unraveling the Powerful Mechanism of Protein Manipulation
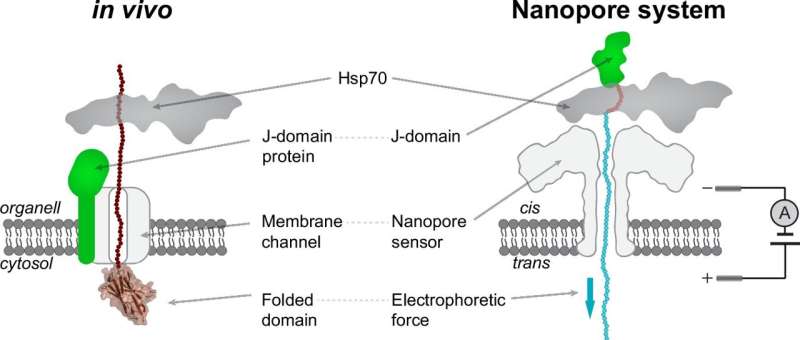
Proteins need to fold into specific three-dimensional shapes to function correctly. Chaperone proteins like Hsp70s assist in the correct folding of proteins, as well as other crucial tasks such as extracting them from aggregates or facilitating their translocation into key cell compartments, like mitochondria.
For decades, there was an intense debate about the mechanism that allows Hsp70 chaperones to drive protein translocation, with two main models proposed. In 2006, a new theory called Entropic Pulling was introduced, which could explain all existing observations for protein translocation and other cellular functions of Hsp70s. However, this theory remained without direct experimental confirmation until now.
The Remarkable Power of Entropic Pulling: How Hsp70 Chaperones Reshape Proteins
The team led by Professor Chan Cao from the University of Geneva used a nanopore single-molecule technique to mimic the in vivo setup of protein translocation. Their results provide clear evidence for the Entropic Pulling mechanism, ruling out the previously proposed Power Stroke and Brownian Ratchet models.
In the Entropic Pulling mechanism, the chaperone, by pulling on the target protein, increases its range of movement, generating a strong entropic force. The researchers estimated the strength of Entropic Pulling to be approximately 46 pN over distances of 1 nm, indicating a remarkably powerful force at the molecular level. This breakthrough not only confirms the Entropic Pulling theory but also establishes nanopore approaches as a powerful tool for exploring the molecular mechanisms of protein action.
