Groundbreaking research has revealed a supercomplex that bridges the critical processes of mRNA translation and decay, providing a deeper understanding of cellular quality control mechanisms. This discovery sheds light on the dynamic interplay between the ribosome, SKI complex, and exosome, which work in tandem to ensure the proper lifecycle of messenger RNA. Messenger RNA serves as the blueprint for protein production, and its degradation is a crucial part of cellular homeostasis. The insights gained from this study have far-reaching implications for our understanding of gene expression and could pave the way for new therapeutic approaches.
Unraveling the Molecular Machinery of mRNA Lifecycle
Messenger RNA (mRNA) is the vital link between the genetic information encoded in DNA and the production of proteins, the building blocks of life. When an mRNA molecule is no longer needed, it must be efficiently degraded to maintain cellular homeostasis.
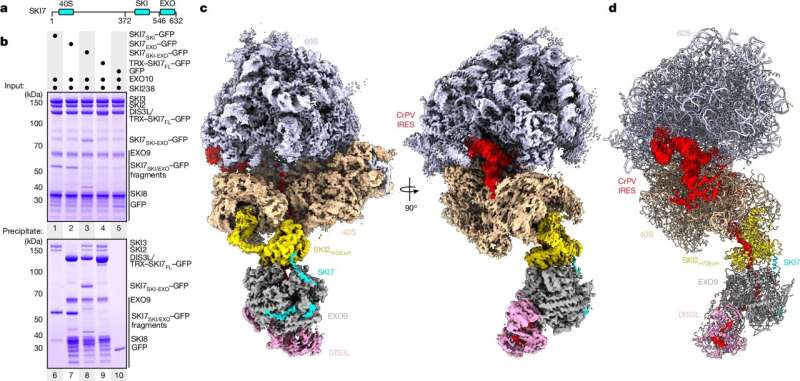
The new study, led by Director Elena Conti and her team at the Max Planck Institute of Biochemistry, has uncovered a remarkable discovery: the various molecular machines involved in mRNA translation and decay are physically linked, forming a supercomplex. This supercomplex consists of the ribosome, the SKI complex, and the exosome, all working together as part of the cell’s quality control machinery.
Unveiling the Intricate Interactions Within the Supercomplex
The functions of the individual complexes have been well understood for years. Ribosomes, often referred to as the cell’s protein factories, translate mRNA into a specific sequence of amino acids, the building blocks of proteins. The SKI complex, on the other hand, plays a crucial role in transporting mRNA to the exosome, which functions like a molecular shredder, degrading the mRNA.
The high-resolution structural data obtained in this study has revealed how these three protein complexes physically interact with one another. It’s akin to a quality control unit in an industrial production line, where the SKI complex attaches to the ribosome, unwinds the mRNA, and transfers it to the exosome for degradation. This process is facilitated by another protein, SKI7, which bridges the interaction between the SKI complex and the exosome.
Insights into Cellular Quality Control and Implications for Therapeutic Developments
The findings of this study provide valuable insights into the cellular quality control mechanisms that ensure the proper lifecycle of mRNA. By physically linking the translation and decay processes, the supercomplex helps to maintain the delicate balance of gene expression and protein production.
Moreover, the study’s focus on the response to mRNA defects, such as collisions between ribosomes during translation, sheds light on how the cell’s quality control system identifies and addresses these issues. This knowledge could have far-reaching implications for the development of new therapeutic approaches, particularly in the field of genetic disorders and diseases associated with mRNA dysfunction.
The advancements in cryo-electron microscopy and the AI-based software AlphaFold have been instrumental in enabling scientists to examine these larger protein machines and understand their intricate interactions. The visual evidence presented in this study exemplifies the power of these technological breakthroughs in unraveling the complex mechanisms underlying cellular processes.
