Clostridium perfringens, a common inhabitant of the human gut, is a notorious pathogen capable of causing serious infections like gas gangrene and food poisoning. In a groundbreaking study, researchers have identified a unique enzyme within this bacterium that plays a crucial role in its ability to degrade and utilize hyaluronan, a key component of the host’s extracellular matrix. This discovery sheds new light on the complex interactions between C. perfringens and its human host, paving the way for better understanding of the bacterium’s virulence mechanisms and potential therapeutic interventions. Clostridium perfringens, Hyaluronan, Extracellular matrix, Gas gangrene, Food poisoning
Unraveling the Secrets of Clostridium perfringens
The human gut is home to a diverse array of microorganisms, collectively known as the gut microbiome. Among these inhabitants is the anaerobic, spore-forming bacterium Clostridium perfringens, which is a major component of the adult human gut microbiota. While most gut bacteria play a beneficial role in maintaining human health, C. perfringens is an opportunistic pathogen capable of causing life-threatening infections, such as gas gangrene and food poisoning.

The virulence of C. perfringens is largely attributed to its production of a wide array of toxins, including the infamous alpha-toxin, which can disrupt the host’s cell membranes and lead to tissue damage and cell death. Additionally, C. perfringens is known to secrete various degradative enzymes, such as hyaluronidases, collagenases, and sialidases, which help the bacterium break down the host’s extracellular matrix and facilitate its invasion and colonization of surrounding tissues.
Unraveling the Mysteries of Hyaluronan Degradation
One of the key components of the host’s extracellular matrix is hyaluronan (HA), a large, non-sulfated glycosaminoglycan. HA plays a crucial role in maintaining the structural integrity and function of tissues, including the gut epithelium. Understanding how C. perfringens interacts with and degrades HA is crucial for elucidating the mechanisms underlying the bacterium’s pathogenicity.
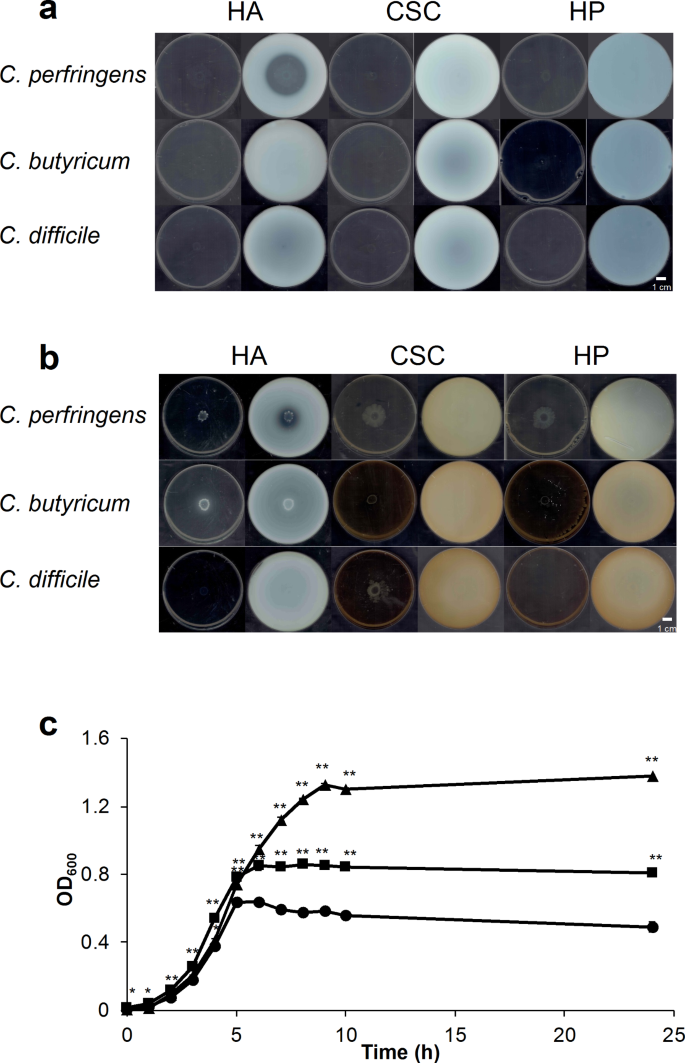
Fig. 2
Previous studies had suggested that C. perfringens possesses several candidate genes encoding hyaluronidases, such as nagH, nagJ, and nagK, which were thought to be responsible for the degradation of HA. However, the true intrinsic HA-degrading enzyme in C. perfringens remained elusive.
Identifying the Key HA-Degrading Enzyme
In a recent groundbreaking study, researchers from Kyoto University set out to uncover the identity of the primary HA-degrading enzyme in C. perfringens strain ATCC 13124. Using a combination of transcriptome analysis, enzyme characterization, and structural modeling, the team made a surprising discovery: the intrinsic HA-degrading enzyme in C. perfringens is not a hyaluronidase, but rather a hyaluronate lyase encoded by the gene hysA.

Fig. 3
The researchers first observed that C. perfringens could degrade and assimilate HA as a carbon source, as evidenced by its growth in nutrient-poor media containing HA. Interestingly, the expression of the hysA gene, which is part of a conserved glycosaminoglycan (GAG) genetic cluster, was significantly upregulated in the presence of HA, while the expression of the previously suspected hyaluronidase genes (nagH, nagJ, and nagK) was unexpectedly low.
Further analysis revealed that the recombinant CpeHysA enzyme, encoded by the hysA gene, exhibited robust HA-degrading activity through a β-elimination reaction, producing unsaturated HA disaccharides. Additionally, the HA-degrading activity in the culture supernatant of C. perfringens was found to be solely attributable to the CpeHysA enzyme, as confirmed by native-PAGE and activity staining.
These findings demonstrate that the intrinsic HA-degrading enzyme in C. perfringens is the hyaluronate lyase CpeHysA, rather than the previously suspected hyaluronidases. This discovery sheds new light on the molecular mechanisms underlying C. perfringens’ ability to degrade and utilize HA as a nutrient source, which is crucial for its survival and pathogenicity within the host.
Implications and Future Directions
The identification of CpeHysA as the primary HA-degrading enzyme in C. perfringens opens up new avenues for understanding the bacterium’s virulence mechanisms and potential therapeutic interventions. The GAG genetic cluster, including the hysA gene, appears to play a key role in the bacterium’s ability to degrade and assimilate HA, as well as other glycosaminoglycans, which are abundant in the host’s extracellular matrix.
By elucidating the molecular details of HA degradation by C. perfringens, this study provides valuable insights into the complex interplay between the pathogen and its human host. The findings suggest that targeting the CpeHysA enzyme or the GAG genetic cluster could potentially disrupt the bacterium’s ability to degrade and utilize host-derived nutrients, thereby compromising its virulence and colonization potential.
Furthermore, the structural similarities between CpeHysA and the well-studied hyaluronate lyase from Streptococcus pneumoniae offer opportunities for comparative studies and the development of novel therapeutic strategies that could be effective against a broader range of pathogenic bacteria.
In conclusion, the identification of CpeHysA as the intrinsic HA-degrading enzyme in C. perfringens represents a significant advancement in our understanding of this opportunistic pathogen’s interaction with the host’s extracellular matrix. This knowledge paves the way for further investigations into the bacterium’s virulence mechanisms and the development of targeted interventions to combat C. perfringens-associated infections.
Author credit: This article is based on research by Tomoya Kumon, Sayoko Oiki, Wataru Hashimoto.
For More Related Articles Click Here
