Researchers have recently found that augmenting the cholesterol in exosomes can greatly improve RNA drug delivery, providing a strong approach to solving this issue concerning siRNA therapy.
The Power of Exosome Unlocking
The RNA interference (RNAi) technology is a powerful tool in the treatment of genetic disorders and cancer by modulating gene expression with high specificity. Yet, one of the major bottlenecks to date has been an effective and safe delivery of small interfering RNA (siRNA) for their clinical application.
The hero of the story is exosomes — naturally occurring nanoscale extracellular vesicles that have gained increased research interest for their potential as ideal vehicles for siRNA delivery. On the other hand, these tiny parcels take place in excellent biocompatibility, stability and long blood circulation time in classical 21244_1_inverseelm1489124590 tissue targeting. However, the major pathway of traditional exosome delivery depends largely on endocytosis, which results in fast degradation of RNA molecules in lysosomes and limits delivery efficiency.
The Cholesterol Connection
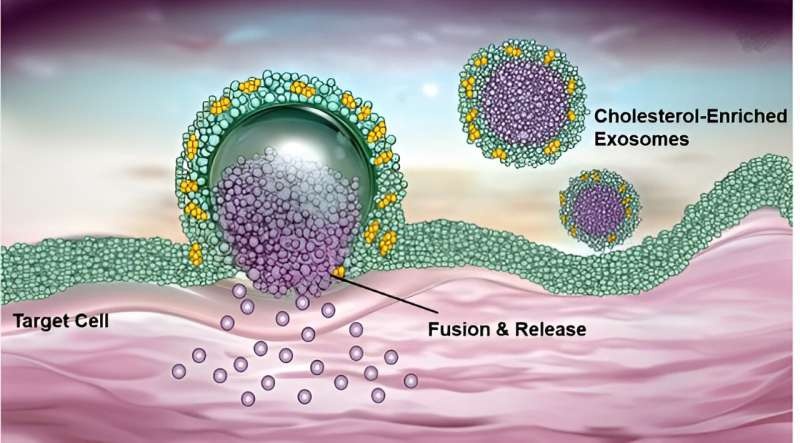
In a paper published in SCIENCE on July 2nd, researchers from the Shanghai Institute of Materia Medica, Chinese Academy of Sciences and Zhejiang University explained the critical role of cholesterol in directing exosomesloaded with RNA drugs to target organs.
The researchers engineered controllable membranes to produce exosomes from different cellular cholesterol environments, for example milk exosomes (in low-cholesterol mammalian cells), and ginger-derived exosome, and a cholesterol-enriched tumorigenic cell line. By systematically profiling different properties of the cholesterol enriched exosome, they showed that an increase in the fractional lipid order at the dividing plane between two liquid domains (i.e. pool 3); enhances the interaction of exosomes with target cell membrane, thus promoting elucidative fusion rather than endocytosis to enter into a cell. This circumventing of the lysosomal degradation pathway is thought to enable a more efficient delivery of the RNA cargo.
In addition, molecular models illustrated that cholesterol-enriched exosomes possessed the high membrane deformability and could get together to more regions of the cell membrane, this made them an robust transport vehicle for delivering siRNA into the cytoplasm via a endocytosis and membrane fusion mechanism.
Conclusion
Thus successful in vitro reconstitution of membrane fusion-mediated cargo transfer by modulating cholesterol content in the exosome membranes forms a basis for an exciting future focusing on the development of safer and more effective gene therapies using this promising approach. These findings could open the door to a future generation of RNA therapeutics for genetic diseases and cancer.
