Osaka university researchers have made a world-first discovery: the strength of species-specific, environmental conditions relevant bacteria that display irregular crystals are dominated by machine learning. This game-changing methodology might transform the diagnostic and therapeutic landscape of bacterial infections, leading to a new vision with an enhanced individualized medicine concept.
The Molecular Road of Antibiotic Resistance
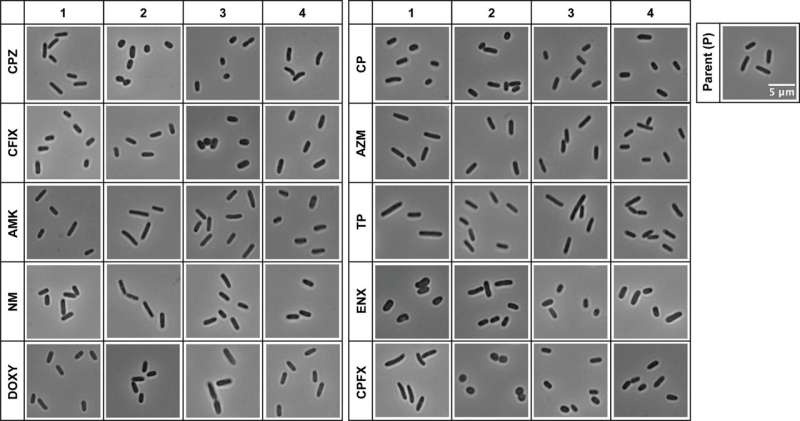
Historically finding antibiotic-resistant bacteria has been a slower process where you grow the bacteria in lab setting and expose them to different drugs and see if any works. But now, scientists at Osaka University have a better idea.
In their study, they found E. coli bacteria exposed to antibiotics develop some specific shapes such as either fatter or shorter compared to a non-resistant strain. These finding suggest that the morphology of bacteria and their susceptibility to this treatment are interwinded.
The researchers trained machine learning algorithms on images of these bacteria, taken using a microscope, to predict which strains are antibiotic resistant or not—with remarkable accuracy and without the need to expose them to any drugs. The discovery may transform how we diagnose and treat bacterial infections, leading to a faster and more specific treatment.
The Genetic Connection
Not satisfied with just watching the physical transformation of antibiotic-resistant bacteria, the researchers went a step further. They then explored further into the genetic basis of this shapeshifting power.
Metric analysis showed that morphological changes in the resistant bacteria are tightly linked with alterations energy metabolism and genes for antibiotic resistance. The bacteria’s genetic makeup is therefore believed to be deeply involved in the ability of these biofilm-forming pathogens to adapt and survive treatment with antibiotics.
This link between bacterial shape, size and resistance to antibiotics could represent a fundamental principle with wide-reaching implications for future new therapeutic strategies. If we were to pinpoint the genetic targets that equip bacteria with this shape-shifting combat trick, we would have a better chance at creating drugs that are both more likely to work and less prone to falling prey to mounting levels of antibiotic resistance.
Conclusion
This discovery by the researchers at Osaka University shows great promise in combating antibiotic resistant bacteria. Through the use of machine learning and knowledge on the genes giving rise to these shape-shifting superbugs, this research highlights potential for new diagnostic tests and treatments that can allow us to anticipate how dangerous antibiotic resistance will become. The possibilities in this groundbreaking area of research are enormous, helping to advance us toward a time where affordable personalized target treatments could be the norm and we could see better health care outcomes across the board.
