Researchers have made a breakthrough in optimizing the expression of recombinant proteins in Chinese Hamster Ovary (CHO) cells, a widely used platform for the production of biopharmaceuticals. By combining vector optimization and cell line modification strategies, the team has developed a novel CHO cell expression system that significantly improves the yield of therapeutic proteins. This research holds immense potential for the large-scale production of crucial drugs, paving the way for advancements in the biopharmaceutical industry. Chinese Hamster Ovary cells, Recombinant proteins, Biopharmaceuticals, CRISPR
Unlocking the Potential of CHO Cells for Biopharmaceutical Production
Chinese Hamster Ovary (CHO) cells have long been the workhorse of the biopharmaceutical industry, serving as a preferred platform for the production of recombinant therapeutic proteins. These mammalian cells possess the ability to properly fold and glycosylate proteins, ensuring that the final products closely resemble their natural counterparts. However, despite the numerous advantages of CHO cells, the expression levels of recombinant proteins have remained a persistent challenge, limiting the large-scale production of critical drugs.
Optimizing Vectors and Modifying Cell Lines: A Synergistic Approach
In a groundbreaking study, a team of researchers has developed a novel CHO cell expression system that addresses the bottleneck of low protein expression. The key to their success lies in a two-pronged approach: vector optimization and cell line modification.
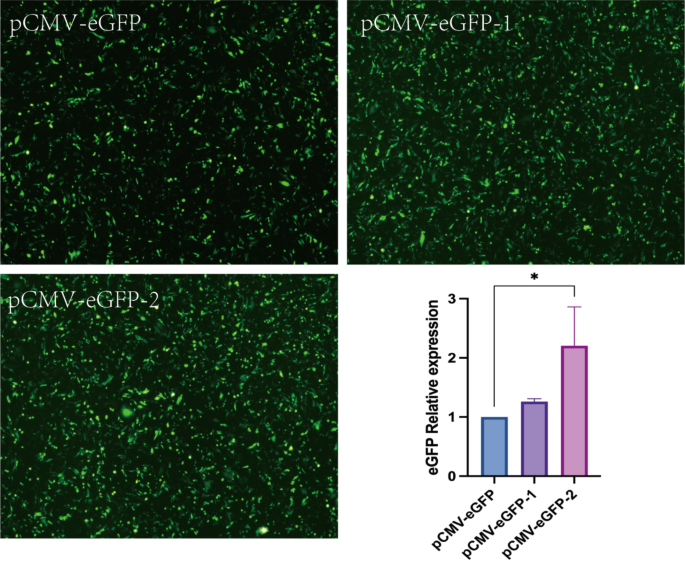
First, the researchers focused on optimizing the vector design. They added regulatory elements, such as the Kozak sequence and the Leader sequence, upstream of the target genes. These sequences play a crucial role in enhancing translation efficiency and improving the expression levels of recombinant proteins.
Next, the researchers turned their attention to cell line engineering. Using the CRISPR/Cas9 technology, they successfully knocked out the Apaf1 gene, which is a key player in the apoptotic (cell death) pathway. By inhibiting this gene, the researchers were able to reduce the proportion of apoptotic cells, thereby increasing the overall survival and protein production capacity of the CHO cells.
Significant Improvements in Protein Expression Levels
The results of this groundbreaking study are truly remarkable. When the optimized vectors were transfected into the Apaf1-knockout CHO cells, the expression levels of recombinant proteins, such as SEAP (Secreted Alkaline Phosphatase) and IL-3 (Interleukin-3), were significantly enhanced compared to the control groups.

Fig. 2
For instance, the stable expression of SEAP in the Apaf1-knockout CHO cells was 1.85- to 2.16-fold higher than in the wild-type CHO cells. Similarly, the stable expression of IL-3 was 1.73- to 1.87-fold higher in the Apaf1-knockout cells. These impressive improvements in protein production lay the foundation for the large-scale manufacturing of therapeutic proteins.
Unlocking the Potential of Difficult-to-Express Proteins
The researchers believe that their novel CHO cell expression system has the potential to unlock the production of even the most challenging recombinant proteins. By combining vector optimization and cell line modification, they have created a versatile platform that can be tailored to the specific requirements of different therapeutic proteins.

Fig. 3
This breakthrough could have far-reaching implications for the biopharmaceutical industry. As the demand for innovative and effective treatments continues to grow, the ability to efficiently produce recombinant proteins at scale becomes increasingly crucial. The researchers’ work paves the way for the development of more potent and accessible biopharmaceuticals, ultimately benefiting patients worldwide.
Ongoing Research and Future Directions
The researchers are not resting on their laurels. They are currently exploring the expression of various difficult-to-express proteins using their novel CHO cell expression system. Additionally, they are investigating the potential of this system to enhance the production of monoclonal antibodies, a class of biopharmaceuticals widely used in the treatment of cancer, autoimmune disorders, and infectious diseases.

Fig. 4
As the scientific community continues to push the boundaries of biopharmaceutical production, this groundbreaking research stands as a testament to the power of innovation and collaboration. By optimizing the expression of recombinant proteins in CHO cells, the researchers have opened up new avenues for the development of life-saving therapies, ultimately benefiting patients and transforming the future of the biopharmaceutical industry.
Author credit: This article is based on research by Junhe Zhang, Chenyang Du, Yue Pan, Zhan Zhang, Ruoyuan Feng, Mengyao Ma, Tianyun Wang.
For More Related Articles Click Here
