Scientists at the Martin Luther University Halle-Wittenberg (MLU) have created a specialized gel that significantly increases the life expectancy, performance and safety of lithium-ion batteries. This novel approach solves the inflammability problems of conventional electrolytes and opens a new chapter leading to safer, cooler, and more reliable batteries.
Taming the Flames
Lithium-ion batteries power everything from our smartphones to electric vehicles because of how versatile and powerful they are. But they have one Achilles’ heel — the toxic combination of material that moves the ions back and forth between the electrodes is essentially a highly flammable electrolyte fluid. Batteries that are damaged like this present a real safety risk as they can either catch fire or explode.
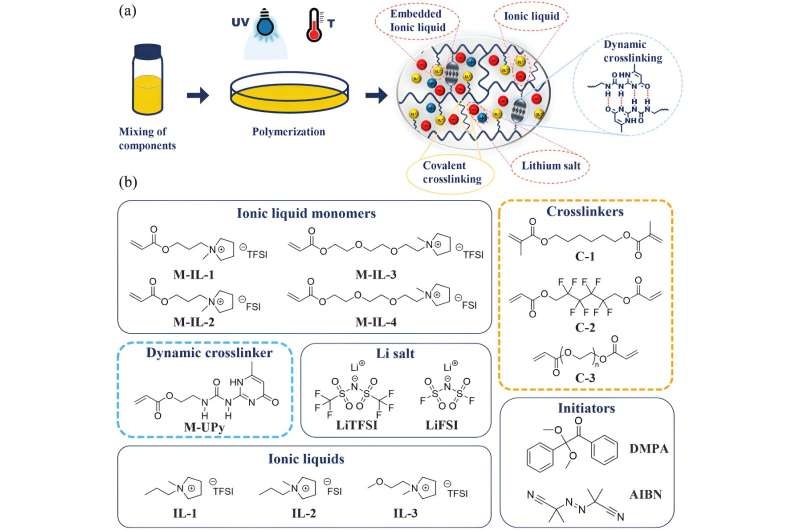
Researchers at MLU have developed an appealing alternative, which is a gel made of a polymer that captures the volume but not the fluidity. This new gel not only improves safety by helping to eliminate leaks and the potential for fire, but it also provides better performance and a longer battery life.
A Breakthrough in Gel Electrolytes
While this is not exactly a new type of battery, having been seen in motorcycle starter batteries for some time. Nevertheless, the mixture of gel electrolytes and lithium ions has proven to be a problem.
Conventional lithium-ion batteries work with a stabilising layer that forms on the electrodes when you charge them for the initial time. This layer is the key to how well and long the battery performs. The MLU researchers have done this by incorporating an ionic scaffold into the molecular chains of the polymer, which enables the formation of this support layer even in a gel electrolyte.
The new gel-based lithium-ion batteries can now operate at voltages more than 5 volts — which felt like a dream just three years ago when the capability of conventional dispersion/solution electrolytes was limited to around 4.2 volts due to their electrochemical stability range for use with graphite anodes and many other positive electrodes. As a result, the improvement not only increases the performance of these batteries but also improves their life-span and perhaps makes them the future of energy storage in the industry.
Conclusion
With this new gel-based lithium-ion battery technology, researchers take a big stride towards the realization of better, more efficient and longer-lasting energy storage options. The flammability issue has been tackled and performances have been improved, allowing MLU researchers to introduce a new generation of stable and environmentally friendly lithium-ion batteries capable of energising anything from our electronic gadgets to the vehicles of tomorrow.
