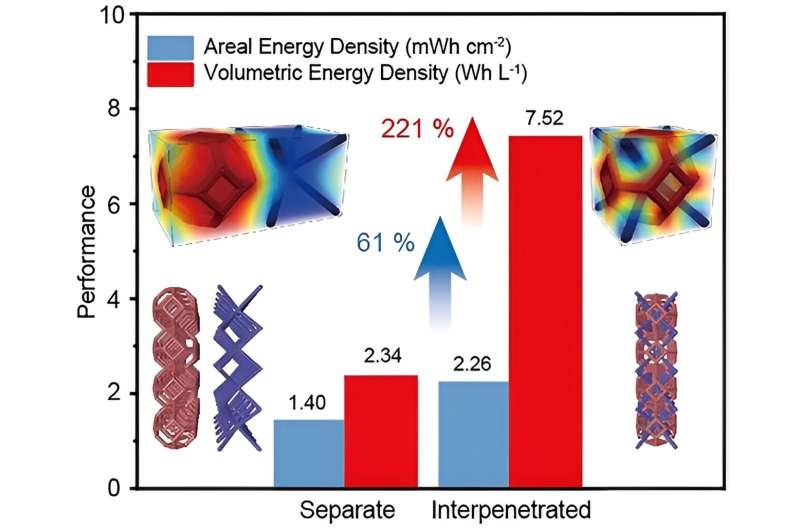
Researchers have developed a novel approach to enhance ion diffusion kinetics in electrochemical energy storage devices, paving the way for improved performance and energy density. The key lies in the use of interpenetrated structures that shorten the ion diffusion path and reduce concentration gradients, addressing the challenges posed by thicker electrodes.
The Issue with Ion Diffusion
In the face of a growing global demand for electrochemical energy storage devices, however, researchers are struggling with a significant challenge: high enough loading to realize capacity and energy density improvements without sacrificing efficient ion diffusion.
The conventional three-dimensional (3D) structured electrodes, with high porosity and low tortuosity, have succeeded to enhance the performance of numerous electrochemical energy storage devices (EESDs). But when the thickness of these 3D-printed electrodes increases, so does the length of ion diffusion path, slowing down the ion diffusion kinetics, and thus decreasing device-level performance.
In an effort to combat this, researchers at the University of California, Santa Cruz have come up with something new: an interpenetrated electrode architecture. This unique approach employs a unit body-centered cubic lattice with Kelvin geometry and two free sublattice electrodes in each unit cell to shorten the travel distance of ions and thus suppress ion concentration polarization.
Creating Interlocking Networks
The scientists used multiple steps to make the IPN electrode structures. They began by using commercial resins as a precursor, and performed the 3D polymerization in stereolithography (SLA) to fabricate polymer interpenetrating films with different number of unit cells.
They then made the polymer substrate electrically conductive by using electroless plating. The polymer surface was sensitized with Sn2+ ions, which underwent a redox reaction with Pd2+ ions to give the immobilization of Pd nanoparticles on the polymer surface. In these, Pd nanoparticles acted as catalytic active sites. Finally, the Pd sites were immersively plated with Ni2+ ions and reducing agent NaH2PO2 to produce a composite layer of conductive Ni-P.
Subsequently, the researchers selectively electrodeposited MnO2/PEDOT composites and metallic zinc on the independently addressable electrodes A and B to form a Zn//MnO2 battery device as a model system.
Conclusion
An interpenetrated electrode structure developed by the researchers represents a promising way to improve ion diffusion kinetics for electrochemical energy storage devices. This new method is effective predominantly in low temperatures, due to the significant limitations of slow ion diffusion by shortening the path of ion diffusion for lowering concentration gradients. Dialing in the size of features and number of interpenetrated units during printing enables a choice between surface area and ion diffusion to optimize performance accordingly, they state. These results highlight the importance of interpenetrated structure to improve electrochemical energy storage and open up new opportunities for higher performance in energy storage.
