Researchers have uncovered a decades-long misunderstanding about the extent of bubble interference in industrial electrochemical processes. The findings reveal that only the area where the bubble contacts the surface is blocked, not the entire area under the bubble. This knowledge could lead to new electrode designs that minimize inefficiencies and reduce energy usage, while also potentially saving on costly catalytic materials. Electrochemical processes play a crucial role in producing fuels, chemicals, and other essential products.
Busting the Bubble Myth: Revealing the True Impact of Gas-Evolving Electrodes
For decades, it has been assumed that the entire area of an electrode shadowed by a bubble is effectively inactivated, reducing the overall efficiency of industrial electrochemical processes. However, new research from a team of scientists, including recent MIT graduate Jack Lake, has challenged this long-held belief.
The researchers discovered that only the area where the bubble actually contacts the surface is blocked from electrochemical activity, rather than the entire area under the bubble. This finding is a significant departure from the accepted theory and could have far-reaching implications for the design of high-performance electrodes.
“The knowledge that the area under bubbles can be significantly active ushers in a new set of design rules for high-performance electrodes to avoid the deleterious effects of bubbles,” says Kripa Varanasi, professor of mechanical engineering at MIT and one of the study’s co-authors.
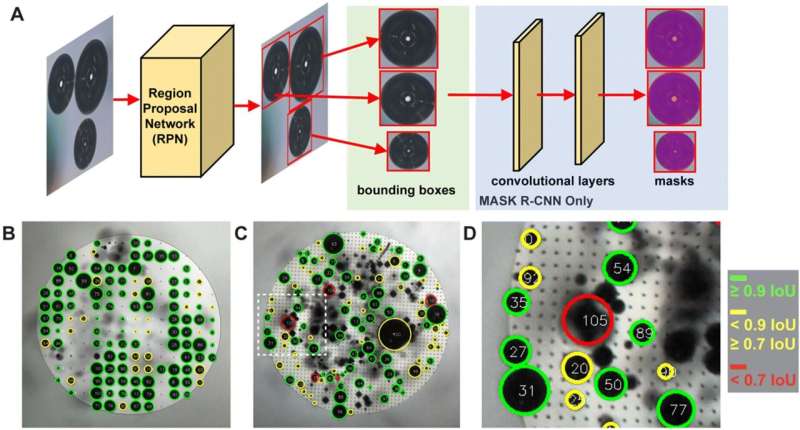
Visualizing Bubble Behavior: How Machine Learning Unveiled the Truth
To test and validate their findings, the research team developed a novel, open-source software tool that uses machine learning to automatically recognize and quantify bubbles formed on electrode surfaces. This tool enabled the researchers to collect vast amounts of data on bubble formation, size, and growth rate, allowing them to correlate visual measures of bubble behavior with electrical measurements of the electrode’s performance.
By using this advanced analytical approach, the team was able to disprove the long-held assumption and demonstrate that only the area of direct bubble contact is affected, while the rest of the surface under the bubble remains active. The researchers also developed a new performance metric, called BECSA (Bubble-Induced Electrochemically Active Surface Area), to more accurately quantify the impact of bubbles on electrode efficiency.
“Creating a program that could deal with different materials and different lighting and reliably identify and track the bubbles was a tricky process, and machine learning was key to making it work,” says Simon Rufer, a graduate student and co-author of the study.
Designing the Future of Electrochemical Processes: Harnessing Bubble Dynamics for Higher Efficiency
The findings of this research could have significant implications for the design of electrodes used in a wide range of industrial processes, from the production of “green” hydrogen to carbon capture and aluminum production.
Instead of focusing solely on minimizing bubble coverage, electrode designers can now shift their attention to minimizing the contact area between bubbles and the electrode surface. This can be achieved by carefully engineering the morphology and chemistry of the electrode, potentially leading to new architectural designs that not only improve overall efficiency but also reduce the usage of costly catalytic materials.
“The insights from this work could inspire new electrode architectures that not only reduce the usage of precious materials, but also improve the overall electrolyzer performance,” Varanasi says. “This could provide large-scale environmental benefits by enhancing the efficiency of these widely used electrochemical processes.”
