Researchers have uncovered the remarkable tactics employed by the bacterium Chlamydia pneumoniae to infiltrate and manipulate human cells. By mimicking the molecular structures of its host, this pathogen hijacks the cell’s own transport machinery to gain entry and replicate within. This captivating discovery sheds light on the intricate interplay between bacteria and their human hosts, paving the way for potential new strategies to combat Chlamydia infections.
Chlamydia’s Molecular Mimicry Tactics
Bacteria that cause diseases, known as pathogens, have developed various strategies to exploit human cells as hosts to their own advantage. A team of researchers from Heinrich Heine University Düsseldorf (HHU) has uncovered the remarkable attack strategies employed by the bacterium Chlamydia pneumoniae.
The researchers describe how C. pneumoniae uses a process called ‘molecular mimicry’ to infiltrate and manipulate human cells. This involves the bacterium imitating the molecular structures of the human cell, allowing it to hijack the cell’s own transport machinery for its own replication. By exploiting the cell’s endocytic process, where the cell membrane curves inward to engulf and transport materials, C. pneumoniae can gain entry and establish itself within the host.
Chlamydia’s Subversive Endocytosis Hijacking
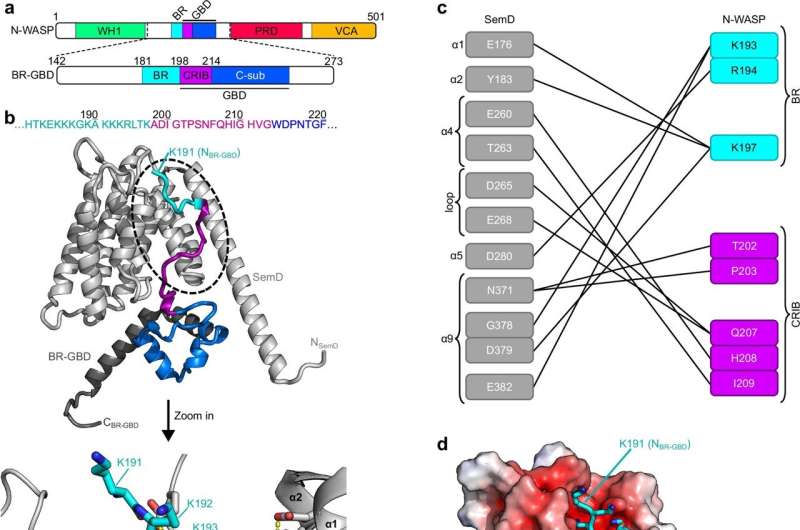
The critical element in the endocytosis process is the cell’s inner actin cytoskeleton, which provides the energy needed for the cell membrane to curve inward and form a membrane-enclosed vesicle. This process is normally triggered by the human protein Cdc42 binding to a specific activator, N-WASP.
However, the researchers have discovered that C. pneumoniae injects a chlamydial protein called ‘SemD’ into the host cell. This SemD protein then binds to the membrane of the forming vesicle, activating the actin cytoskeleton and causing the plasma membrane to fully engulf the entire bacterium. Remarkably, SemD is specifically designed to mimic the structure of Cdc42, allowing it to bind even more effectively to N-WASP than the normal human activator. The bacterium even goes so far as to displace Cdc42, ensuring its own protein can take control of the endocytic process.
Implications and Future Prospects
This groundbreaking research has shed light on the intricate and subversive mechanisms employed by C. pneumoniae to infect human cells. By leveraging structural and functional mimicry, the bacterium is able to hijack the host’s own cellular machinery to its advantage, facilitating its entry and replication within the human body.
The researchers are now hopeful that this newfound understanding of the Chlamydia-host interaction can pave the way for the development of agents that can prevent the specific interaction between the bacterial and human proteins, potentially blocking C. pneumoniae infections in the future. This discovery highlights the ongoing arms race between pathogens and their hosts, and the importance of continuing to unravel the complex biological mechanisms that underlie infectious diseases.
