Dinosaur collagen has been detected from an 80-million-year-old Brachylophosaurus canadensis and the academics described the “secret” of its preservation. The find casts doubt on what we thought we knew about the average life of peptide bonds and provides some answers about why this longstanding biopolymer has the staying power that it does.
Atomic Defense of Collagen
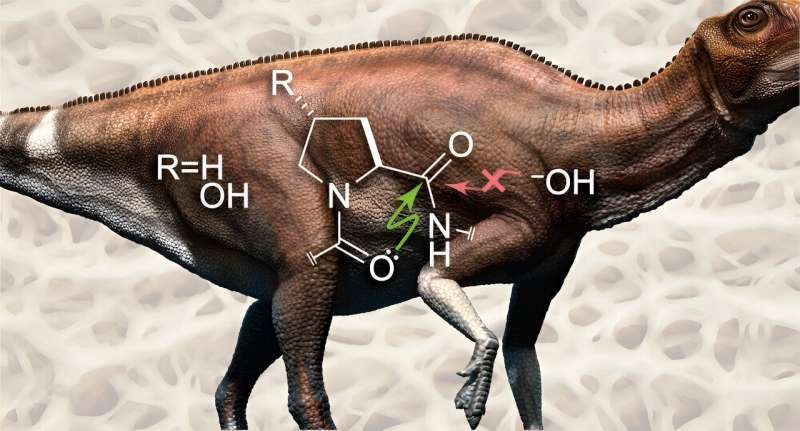
The reason for collagen’s persistence could be an atomic-level quirk that keeps it safe from the clutches of time. Collagen has a large repeating sequence, known as Gly-X-Y (right), and researchers from MIT found that the carbonyl oxygen in the peptide bonds of these repeats could offer up its pair of electrons to the neighbouring peptide bond’s carbonyl group.
These schemes of electron-sharing generate a barrier and block the water molecules to tresspect it, causing hydrolysis (the breakdown of peptide bonds). Peptide bonds typically half-life in ~500 years, but with this next-level atomic protection system, collagen has the power to last millions of years.
The researchers showed this by making two forms of collagen — the usual trans form, and a twisted cis form. The researchers discovered that the trans form – which can undergo this electron-sharing reaction with water – was unaffected by an attack of water, whereas the cis form could be destroyed rapidly by hydrolysis.
How Collagen, the Unbreakable Structure
Collagen is the most abundant protein in animals, present not solely in bones however collectively among your pores and skin, muscle tissue, tendons, and ligaments. A strong, fibrous protein that exists in a triple helix structure as the structural scaffold of our bodies.
That unbroken triple helix end to end is so unique that it is what makes collagen one of a kind from other proteins. Whereas other proteins like those holding together alpha helices and connected by sequences that continue to exist in an exposed form leave weak points, collagen does not. Only the uninterrupted triple helix and the atomic-level defense against water allowed collagen to withstand time, now preserved even in dinosaur fossils.
While past hypotheses had posited that the bones’ near-total dehydration may have helped to maintain collagen, the new conclusion that the molecular-discipline step is an important component of why this ancient biological material sticks around for so long.
Conclusion
In paleobiology this is a remarkable finding, the atomic-level defense that helps collagen last much longer than one should ever expect of such delicate peptide bonds. The results provide new insights into the exceptional preservation of dinosaur fossils and offer wider implications for how durable proteins are over deep time. Decoding the mysteries of these ancient biomolecules could indeed provide us with new methods by which to conserve and even analyze the fossil remains of life from long past geological ages.
