Researchers at TU Delft and the Max Planck Institute have developed a groundbreaking new class of DNA nanopores that can open, close, and adjust their diameter on demand. This revolutionary breakthrough offers unprecedented possibilities for biomedical applications, including the controlled and size-selective delivery of macromolecules, a crucial step towards more advanced targeted drug delivery systems.
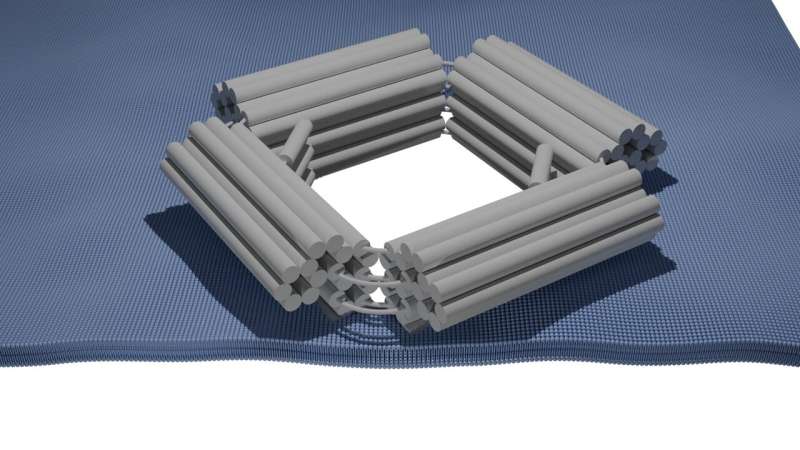
Adaptive DNA Nanopores
Although traditional DNA origami nanopores have become a powerful tool in the biophysics and biotechnology field, their application as an improved means for delivering larger therapeutic molecules has been limited. To overcome that challenge, the TU Delft and Max Plack Institute researchers have developed a novel type of DNA nanopores called ‘MechanoPores’, which carries an enlarged 30-nanometer wide opening instead of the standard 4-5 nanometers.
This is because the single-stranded DNA molecules within their lattice are “flexible”: changing in shape and, therefore, allowing for pores that can switch between open and closed states. The pores can be propped open by the addition of DNA strands with complementary sequences that are larger and harder to get through. On the other hand, attaching single-stranded DNA molecules that are complementary to those in the gain of function inserts on the outside can close up a pore and control transport precisely.
Tailoring Molecular Transport
Now, the researchers have not just achieved switching nanopores between open and closed states in a reversible way but also demonstrated unprecedented control over their diameter. The pore sizes will allow a molecule to pass through but not bind because the pore is too small.
A second critical piece of the puzzle was being able to get the MechanoPores to embed in cell membranes, something no one has been able to accomplish until now. Proven by fluorescence imaging, the achievement is an important milestone on the path to practical use of these nanopores in real-life biomedical applications, like personalized medicine.
For the Selective Transport of Molecule
Although the research has shown that the MechanoPores can achieve size-selective transport of molecules, the ultimate goal for Hanson and Hall is to go one step further by applying mechanical pressure to differentiate between different types of molecules along their molecular composition, not only against their size.
In a promising next step that takes advantage of the nanopores’ ability to tell apart proteins that are almost the same size, such precision could enhance molecular diagnostics and even make targeted drug delivery more precise. These DNA nanopores can be optimized for the selective transport of targeted biomolecules, which could potentially enable the individualized medicine that accounts for personal patient variations as cancer is a disease with heterogeneous characteristics in patients.
The future of controlled drug delivery looks bright with these new innovative nanodevices can serve as a strong contender for applications of such complex solutions, exhibiting the immense power and potential of nanotechnology in revolutionizing how we think about advanced biomedical dilemmas.
