Researchers from the University of Oregon have developed a novel 3D printing process that incorporates fluorescent ring-shaped molecules, creating intricate glowing structures with potential medical applications. This groundbreaking discovery paves the way for more effective tracking and monitoring of biomedical implants within the body.
The Cleverest Yet The Most Basic Solution
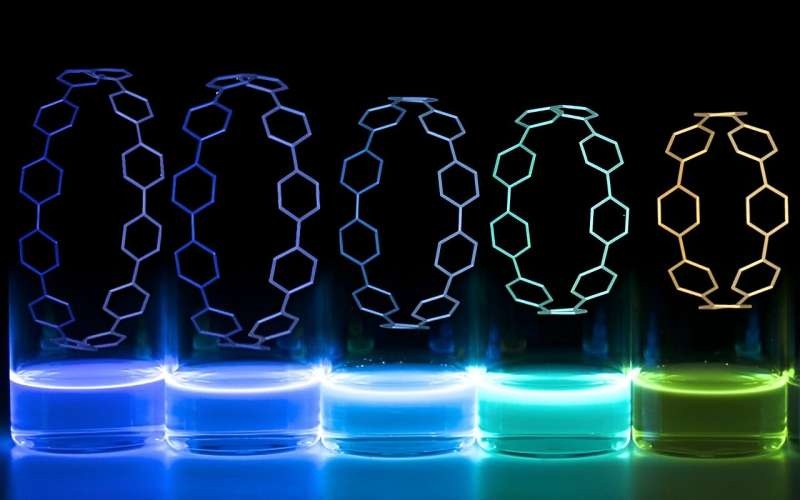
For example, think about medical implants that light up so they can be seen and tracked within the body over time. And that’s exactly what a team of researchers at the University of Oregon did with an impressive coordination between their engineering and chemistry labs.
Adding the fluorescent ring-shaped molecules, called nanohoops, to the 3D printing material is an easy task. That gives rise to glowing, detailed structures that would facilitate the fabrication of novel biomedical implants. A major advantage of this strategy is that it achieves a solution to a design problem that has plagued researchers for years — that by using their approach, they can differentiate between the implant and the surrounding cellular tissues.
A happy accident during casual conversations between an engineering professor, Paul Dalton, and a chemistry professor, Ramesh Jasti indicated what might be possible if they coupled forces. Dalton is an expert on innovative 3D printing techniques, and Jasti’s lab develops nanohoops with unusual fluorescence.
Simplifying Datasets — Merging Complex Field
Although the result seems simple enough, it was anything but clear sailing to arrive at this successful conclusion. It has taken both Dalton’s and Jasti’s labs years to get their methods just right, encountering many roadblocks along the way.
It overcomes a limitation of 3D printing by offering the capacity to print large shapes, at impressive resolutions, and with no need for digital blueprints or extra post-print processing. That has allowed the team to design mesh scaffolds for a range of biomedical implants, including technology as diverse as wound-healing patches, artificial blood vessels, and nerve regeneration structures.
In the case of Jasti’s lab, they have been developing nanohoops, carbon-ring-like molecules that have a variety of valuable characteristics (DOI: 10.1021/ar700107p). Now, the big draw of these nano hoops is that they fluoresce very intensely when excited with ultraviolet light, shining in different colors depending on their size and arrangement.
When these two also apparently unrelated fields collided, the researchers were surprised to find that the framework was not only working. “Fabricating nano hoops is hard, and melt electrowetting is also really hard to do — but thanks to an inventive collaboration between physical chemists and materials researchers, what we developed was quite simple despite these two seemingly different avenues colliding,” explained Harrison Reid, a graduate student involved in the study.
Conclusion
Introducing 3D-printed tissues is a major milestone in medical/new tech developments. Using the nano hoops as part of the material for 3D printing, it is possible to implant more easily and accurately biomedical implants inside our bodies.
The scope of applications includes wound healing and nerve regeneration, among others. While the team is still tweaking and testing their material, the implications in the medical world are fascinating. A fortuitous pairing of the UO engineering and chemistry labs has culminated in a finding that could be a game changer in future healthcare applications.
