Medication non-adherence is a significant challenge in kidney transplantation, potentially leading to graft rejection and other adverse outcomes. Researchers have developed a new tool called the Kidney AlloTransplant Immunosuppressive Therapy Adherence Questionnaire (KATITA-25) to identify patients who may be predisposed to non-adherence even before their transplant. This cross-cultural validation study, conducted in Spain, shows that the Spanish version of KATITA-25 is a reliable and valid instrument for assessing the risk of non-adherence in kidney transplant candidates. By identifying high-risk patients early, healthcare providers can implement targeted interventions to improve medication adherence and optimize transplant outcomes. This research fills a crucial gap in clinical assessment tools for Spanish-speaking populations and could have far-reaching implications for kidney transplant care worldwide.
Tackling Non-Adherence in Kidney Transplants
Kidney transplantation is a life-saving procedure, but the success of the transplant depends heavily on the patient’s adherence to immunosuppressive medications. Non-adherence to these medications can lead to graft rejection, organ failure, and other serious complications. Unfortunately, non-adherence is a widespread issue, with up to one-third of kidney transplant recipients being non-adherent to their medications.
A Novel Approach to Predicting Non-Adherence
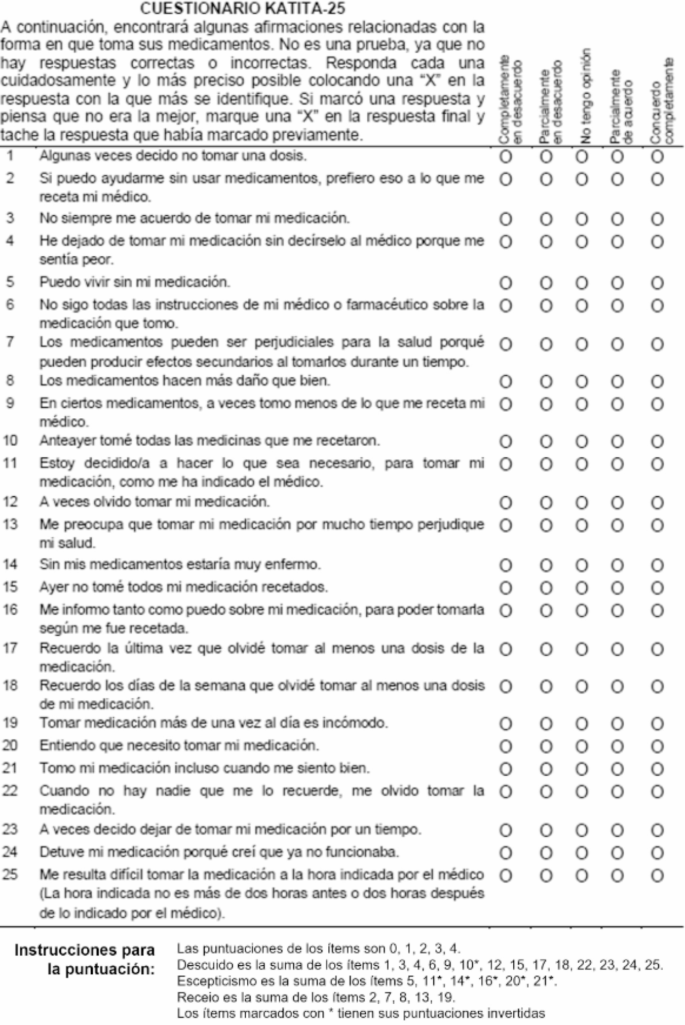
To address this challenge, researchers developed the Kidney AlloTransplant Immunosuppressive Therapy Adherence Questionnaire (KATITA-25). Unlike traditional medication adherence questionnaires that focus on past non-compliance, KATITA-25 takes a novel approach by assessing the psychological factors that may influence a patient’s propensity for non-adherence, even before the transplant.
Validating KATITA-25 in Spanish
The recent cross-cultural validation study, conducted in Spain, aimed to evaluate the reliability, validity, and applicability of the Spanish version of KATITA-25. The researchers followed a rigorous translation and adaptation process to ensure the linguistic and conceptual equivalence of the Spanish version.
The study involved 163 kidney transplant candidates from four different healthcare centers in Catalonia, Spain. The researchers found that the Spanish version of KATITA-25 demonstrated good internal consistency, test-retest reliability, and construct validity. These results closely mirrored those observed in the original Brazilian Portuguese version, confirming the cross-cultural validity of the instrument.
Implications for Kidney Transplant Care
The availability of the Spanish version of KATITA-25 is a significant development, as it fills a crucial gap in clinical assessment tools for Spanish-speaking populations. By identifying patients at high risk of non-adherence before their transplant, healthcare providers can implement targeted interventions to improve medication adherence and optimize transplant outcomes.
These interventions may include:
– Personalized education and counseling on the importance of medication adherence
– Strategies to address specific barriers and concerns related to immunosuppressive medications
– Closer monitoring and support for high-risk patients during the pre-transplant and post-transplant periods
Kidney transplantation is a global healthcare concern, and the prevalence of medication non-adherence underscores the need for effective tools to identify and address this issue. The successful cross-cultural validation of the Spanish KATITA-25 questionnaire represents an important step in enhancing pre-transplant evaluation and improving transplant outcomes for Spanish-speaking patients worldwide.
Author credit: This article is based on research by Luana Cristina Lins de Medeiros Oliveira, Guillermo Pedreira-Robles, María José Pérez-Sáez, Marta Crespo, Anna Bach-Pascual, Sandra Rubio-Paez, Tania Curado-Soto, Alicia Rovira-Algara, Edoardo Melilli, Javier Jerez-Roig, Ester Oriol-Vila, Cristina Quintana Reyes, Maribel Diaz Jurado, Rand Randall Martins, Francisca Sueli Monte Moreira, Antonio Gouveia Oliveira.
For More Related Articles Click Here
