Cerebral malaria, a severe complication of malaria caused by the Plasmodium falciparum parasite, can lead to devastating neurological consequences in patients. In a groundbreaking study, researchers have developed a novel in-vitro model using human induced pluripotent stem cell (iPSC)-derived neuronal cultures to shed light on the underlying mechanisms of this debilitating condition. The findings reveal that the malaria toxin, hemozoin, can activate inflammatory pathways, induce DNA damage, and trigger neurodegenerative processes in neurons – providing crucial insights into the complex pathogenesis of cerebral malaria.
Unraveling the Mysteries of Cerebral Malaria
Malaria, a deadly infectious disease caused by Plasmodium parasites, remains a significant global health challenge, with an estimated 249 million cases reported in 2022. One of the most severe complications of malaria is cerebral malaria (CM), which can result in neurological sequelae in up to 30% of survivors. Understanding the underlying mechanisms of CM has been a long-standing challenge, as studies have been hindered by limited access to human brain tissues and the inability of the Plasmodium falciparum parasite to infect rodent models.
Harnessing the Power of Stem Cells to Model Cerebral Malaria
To overcome these limitations, the researchers in this study utilized human induced pluripotent stem cells (iPSCs) to generate neuronal cultures, which serve as a valuable in-vitro model for investigating the molecular mechanisms underlying CM. By exposing these neuronal cultures to the malaria toxin, hemozoin (HMZ), the researchers were able to recapitulate key aspects of the disease pathogenesis observed in CM patients.

Inflammation, DNA Damage, and Neurodegenerative Pathways
The study’s findings reveal that exposure to HMZ triggers a cascade of events in the neuronal cultures. Firstly, the researchers observed an elevated secretion of pro-inflammatory cytokines, such as interleukin-1β (IL-1β), IL-8, interferon-gamma (IFN-γ), and IL-16, which are known to be associated with severe forms of malaria. Additionally, the researchers identified the activation of inflammatory pathways, including the mitogen-activated protein kinase (MAPK) cascade and pathways linked to neurodegenerative diseases, such as Alzheimer’s disease.
Remarkably, the study also revealed that HMZ exposure induces DNA damage in the neuronal cultures, as evidenced by increased levels of the DNA damage marker, γH2AX. This finding suggests that the malaria toxin can directly compromise the integrity of neuronal DNA, potentially contributing to the observed neurological deficits in CM patients.

Fig. 2
Activation of the p38 MAPK Pathway
Further investigation into the DNA damage response pathways revealed that HMZ-exposed neurons exhibited an upregulation of ATM, ATR, CHEK1, and CHEK2 – key molecules involved in DNA damage checkpoints. Interestingly, the researchers found that the p53 signaling pathway, which is typically activated in response to DNA damage, was not significantly affected. Instead, the study suggests that HMZ may preferentially activate the p38 MAPK pathway, which has been previously linked to inflammatory responses and neurodegeneration in the context of malaria.
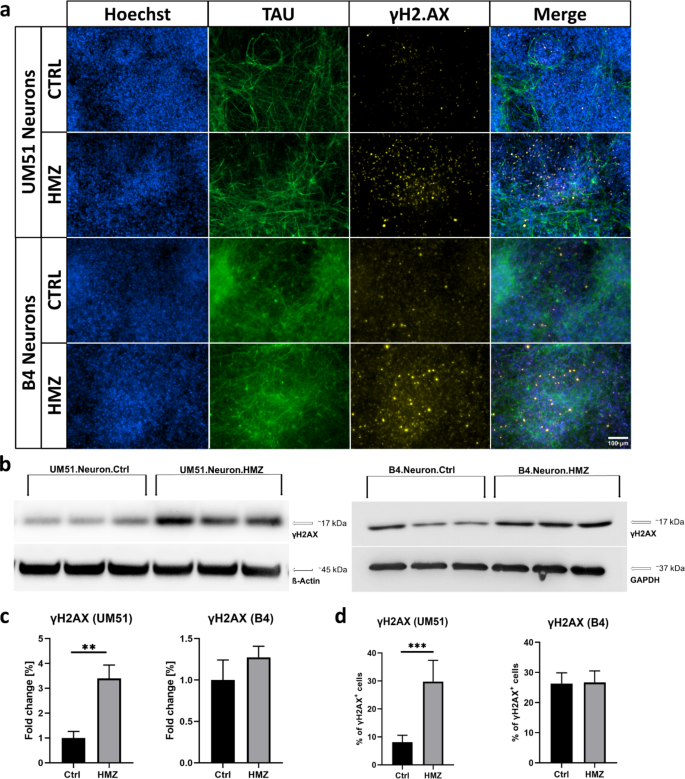
Fig. 3
Implications and Future Directions
The findings of this study have far-reaching implications for our understanding of CM and its potential links to neurodegenerative disorders. By establishing an iPSC-derived neuronal model that recapitulates the hallmarks of CM, the researchers have created a valuable tool for further exploration of the disease mechanisms and the development of targeted interventions.
The study’s insights into the role of inflammation, DNA damage, and the activation of specific signaling pathways, such as p38 MAPK, open new avenues for future research. Exploring the interplay between these processes and their long-term consequences on neuronal function and survival could shed light on the neurological sequelae observed in CM survivors.

Fig. 4
Furthermore, the potential connections between CM and neurodegenerative diseases, as suggested by the upregulation of Alzheimer’s disease-associated pathways, warrant further investigation. Understanding these shared molecular mechanisms could lead to the identification of common therapeutic targets and the development of novel strategies to address both CM and neurodegenerative disorders.
In conclusion, this groundbreaking study has leveraged the power of human iPSC-derived neuronal cultures to unravel the complex mechanisms underlying cerebral malaria. By revealing the pivotal role of the malaria toxin, hemozoin, in triggering inflammatory, DNA damage, and neurodegenerative responses, the researchers have laid the foundation for a deeper understanding of this devastating condition and its long-term neurological consequences.
Author credit: This article is based on research by Abida lslam Pranty, Leon-Phillip Szepanowski, Wasco Wruck, Akua Afriyie Karikari, James Adjaye.
For More Related Articles Click Here
