Researchers at the University of California San Diego have developed a groundbreaking technology that can create temporary openings in the nucleus of a cell without damaging the cell’s outer membrane. This remarkable innovation could revolutionize gene therapy, drug delivery, and precision medicine. Learn how these nanopillars work their magic and unlock new possibilities in the field of cellular manipulation.
Traversing the Nuclear Fortress
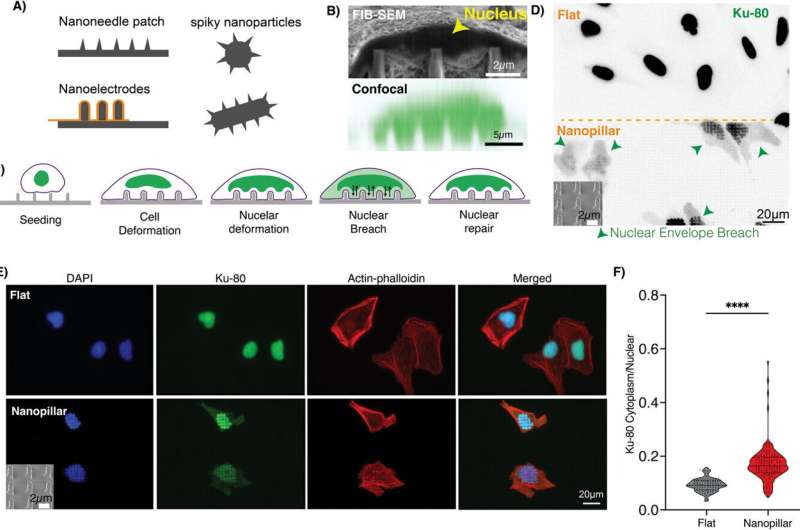
Think about trying to punch a hole through the yolk of a raw egg without puncturing the fragile white. That may sound like some kind of science fiction nonsense, but researchers at UC San Diego pulled off a similar stunt using living cells. Their new technology has been created to penetrate a cell, where the DNA is protected within its nucleus, without harming the external cellular layer.
And the nucleus is a massive impenetrable fortress around our genetic code, letting only specific molecules in through highly regulated channels. “Nothing goes into the nucleus easily,” said Zeinab Jahed, a professor of chemical and nanoengineering in the Aiiso Yufeng Li Family Department of Chemical and Nano Engineering at UC San Diego and the senior author of the study. Manipulating the nuclear membrane to deliver drugs and genes has been a major problem.
The Nanopillar Solution
To address this problem, Jahed and the nanoengineering Ph. D. student Ali Sarikhani, created a non-invasive fix. From the Genus Novarc, They designed a nanometrical array of nanopillars represented by a short cylinder or column. The cell then wraps its nucleus around these nanopillars and deforms its membrane. This curvature in turn led to the transient formation of minimal, self-sealing pores in the nuclear membrane, without harming the plasma membrane.
“Because we can selectively make these tiny holes in the nuclear membrane to expose their nuclei, this is an exciting advance because the functional cell remains completely readable,” Jahed said. They seeded the nanopillars with cells that had taken up a fluorescent dye (such dyes normally are confined to compartments within a cell, and here were trapped in the cell nucleus), then watched as the dye escaped through holes created by one of the two methods — never leaving limits defined by the cell membrane. This suggested that only nuclear membranes had been breached.
Breaking Open New Frontiers in Precision Medicine
The promise of the technology is extensive. The results of the study, which was published in Advanced Functional Materials, could have important implications for gene therapy and other forms of precision medicine where genetic material must be delivered directly to the nucleus or to natural drugs activated only when they are needed.
The team now plans to delve into the biology that drives this phenomenon to continue enhancing the platform for optimal efficacy and safety in future clinical studies of the engineered AAV used in a nuclear approach. Given this, understanding these nuances will be important in how we leverage the platform for a clinical indication and ensure it can do so safely and efficaciously using these complex animals that need to deliver genetic material into the nucleus,” Jahed said.
This revolutionary progress is a game changer and it’s hard to be optimistic about what is now possible, in the future of targeted cellular interventions and personalized medicine. This ability to make non-lethal cuts at the nucleus paves the way for new treatment approaches in gene therapy, drug delivery, and more.
