New data from first accountable measurements help settle more than 40-year-old scientific debate, set stage for new discovery associations with cancer treatment. The results reveal, for the first time, detailed atomic-scale chemistry of serine hydroxymethyltransferase (SHMT), a metabolic enzyme important in cell division and cancer development.
Deciphering the Catalytic Mechanism of the Enzyme
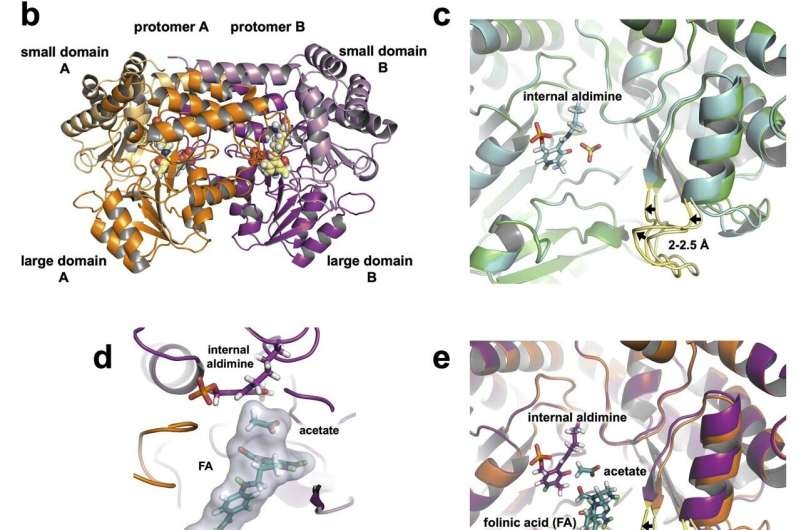
In order to fully grasp the function of the SHMT enzyme, researchers utilized a unique set of neutron and X-ray crystallography capabilities.
The exact catalytic mechanism along with the roles of specific amino acid residues in the enzyme’s active site had been a matter of debate over decades. But the long-sought answer was not directly observable until high-fidelity neutron data were obtained from scientists with the DOE Office of Science’s Spallation Neutron Source (SNS) at Oak Ridge National Laboratory in August followed by additional more uniquely sensitive SNS and High Flux Isotope Reactor experiments in October.
The researchers determined that a single amino acid, glutamate, is vital to the activity of SHMT. The neutron results indicated how this glutamate switches between its acidic and basic forms during the course of accepting protons from the enzyme, thereby enabling it to flow protons in a direction required for the various biological functions of MetY in methionine one-carbon metabolism.
Exploiting The Metabolic Vulnerabilities of Cancer
SHMT is one of the core enzymes that support one-carbon metabolism, which cancer cells highjack to amplify cell growth and division. The authors have provided a comprehensive picture at the atomic level of how SHMT works correctly, offering a blueprint for designing inhibitors that could shut down this metabolic circuit and put the brakes on cancer growth.
Traditional cancer therapies tend to cause broad side effects, whereas blocking enzymes such as SHMT at an earlier stage of the metabolic pathway may be more specific and less toxic. Specifically, the researchers stress that this understanding, when combined with artificial intelligence technologies, could fast-track the design of personalized drugs that can target and destroy cancer cells without harming normal tissues.
Conclusion
For the first time, scientists at Oak Ridge National Laboratory have conducted neutron structural analysis of a human enzyme — and discovered how it works. The act of discovering something isn’t as easy as turning a key into a lock. In revealing the atomic-scale functioning of this enzyme, the team offers vital information to inform more directed and efficient cancer therapies. The finding demonstrates the breakthroughs that neutron-based research can bring in solving one of the toughest diseases of our time.
