In order to solve the energy crisis, one new manufacturing method that uses abundant low-cost metals to produce safe and efficient hydrogen production catalysts has been proved as a cost-effective way of producing high-quality fuels.
The promise of carbon nitride
Carbon nitride (g-C3N4) has been extensively investigated as an active semiconductor, but the low charge mobility and small surface area of g-C3N4 are bottlenecks for its effective catalysis for HER and OER in water electrolysis.
Researchers at China’s Xi’an University of Architecture and Technology tackled that problem head-on, offering a new approach to these challenges. Targeted doping and interfacial coupling, modern techniques have afforded two highly active Schottky junction electro-catalysts, B-C3N4@Fe3C and S-C3N4@Fe3C for significantly promoting redox kinetics of g-C3N4 hollow nanotubes.
The secret of their success, as you might expect, is in carefully controlling the semiconducting properties of g-C3N4 by precisely doping it with boron (B) and sulfur (S). This gives rise to the n-type and p-type band structures, respectively, which is highly desirable for building the surface-functionalized Schottky junction catalysts.
The Power of Schottky Junctions
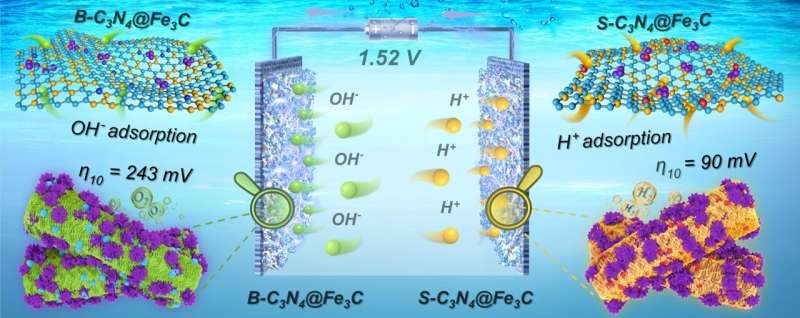
The Schottky junction formed between Fe3C and g-C3N4 in the Schottky junction catalysts adjusted the band position of g-C3N4 to optimize the energy level, changing the surface charge distribution so as to enrich OH- and H+ on solid-liquid reaction interface.
The rational design of Schottky junctions results in excellent catalytic performance for both HER and OER with alkali solution on the B-C3N4 @ Fe 3 C and S-C3N4 @ Fe 3 C catalyst. Based on the obtained results, the authors demonstrated that B-C3N4@Fe3C||S-C3N4@Fe3C can perform water electrolyses efficiently at 10 mA cm-2 only by applying a low voltage of 1.52 V, which confirmed their outstanding electro-catalytic performance and satisfaction stability in long-term alkaline water splitting application.
The work represents a major advance towards a future of water electrolysis with non-precious metal catalysts, which will be not only cheaper but also more sustainable.
Conclusion
In particular, the B-C3N4@Fe3C and S-C3N4@Fe3C are developed as Schottky junction catalysts composed of non-oxide, also notable as non-noble metal for hydrogen production through water electrolysis. Through fine tuning of the semiconductor properties and interfacial charge distribution, we have shown that these catalysts display exceptional catalytic performance and stability, paving a way for scaling up clean hydrogen production to tackle the energy crisis while promoting hydrogen as a green fuel.
