Aiming to help overcome this problem, researchers have now designed a new water electrolysis system that uses non-precious metal catalysts and is capable of producing high-purity hydrogen efficiently and economically.
Unlocking Carbon Nitride FULL TEXT
A C 3 N 4 has been investigated as a semiconducting material, whereas its poor charge mobility and low specific surface area are responsible for the low catalytic performance among various catalysts in hydrogen evolution reaction (HER) and oxygen evolution reaction (OER). Chinese researchers at Xi’an University of Architecture and Technology found a creative way to deal with this problem.
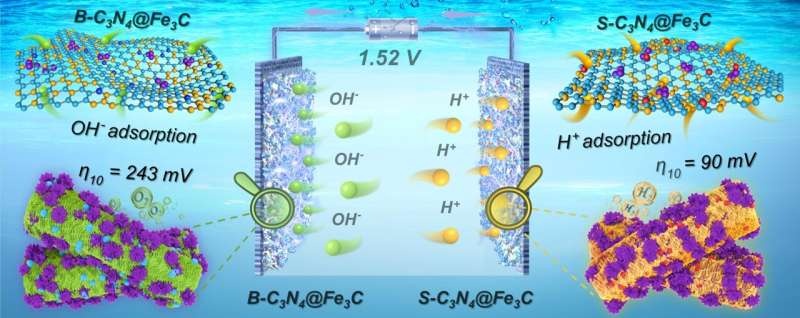
The team proposed a targeted doping and interfacial coupling strategy for activating of Schottky junction electrocatalysts (B-C3N4@Fe3C and S-C3N4@Fe3C). Such an approach makes them able to tune the semiconducting property of g-C3N4 accurately also which brings n- and p-type band structures. Therefore, this enables creating the g-C3N4 with well-matched energy level and modifying the change of electron distribution in the interface area between g-C3N4 and Fe3C can provide better carrier transport pathways for devising surface functionalized Schottky junction catalysts.
The novel strategy increases the concentrations of hydroxide ions (OH-) and hydrogen ions (H+) at the solid-liquid reaction interface uniformly, which is beneficial for promoting both catalytic activity and selectivity towards HER and OER under alkaline conditions.
Outperforming Precious Metals
Water electrolysis traditionally uses precious metal catalysts, such as platinum and iridium, to drive the HER and OER. Nevertheless, the high cost as well as limited abundance of these materials have proved major obstacles in the scale-up of water electrolysis for hydrogen production.
Furthermore, the B-C3N4@Fe3C and S-C3N4@Fe3C catalysts prepared by the research team exhibit excellent performance even at relatively low voltages (1.52 V) for 10 mA cm-2 in water electrolysis. This value indicates their attractive intrinsic electrocatalytic activity and decent long-term stability when employed as an alkaline water splitting anode.
In addition, the application of less expensive non-precious metals to these catalysts such as boron (B) and sulfur (S) for doping, and incorporation of iron carbide (Fe3C) lowers the cost of waster electrolysis process overall. This makes the technology more feasible and scalable, thus allowing for wider adoption to accelerate global energy solution.
Conclusion
The applicability of Schottky junction characteristic for non-precious metals has gained more attention as this would be a key advancement in the water electrolysis catalysis with respect to hydrogen production. The unique properties of carbon nitride coupled with the strategic design for an optimized interfacial charge distribution, has provided a solution that is both efficient and economically viable to accelerate the transition into a sustainable energy future.
