In a groundbreaking move, researchers at SMU have developed a cutting-edge tool called SmartCADD that combines artificial intelligence, quantum mechanics, and computer-assisted drug design techniques to accelerate the drug discovery process. This innovative platform is poised to revolutionize the way we approach the complex task of finding new therapeutic compounds, with the potential to significantly reduce timelines and unlock new avenues for drug development. Drug discovery and quantum mechanics have never been more intertwined.
AI and Quantum Mechanics: Next Level of Excellence is Achieved
Drug discovery remains akin to complexity of solving a jigsaw puzzle with interlocked pieces: only the right-sided shape combined chemically in three-dimensions properties engender, stimulating protein sites in our bodies minimizing specific effects and physiologies desired. Researchers have long struggled with this problem, because the process is painstakingly slow and incredibly complex.
That’s where SmartCADD comes in, a game-changing tool developed by the chemistry and computer science departments at SMU. Through the use of artificial intelligence, quantum mechanics and sophisticated computer-assisted drug design tools, this open-source virtual platform can screen billions of chemical compounds in a single day — slashing the time it takes to identify leading drug candidates. That is a tremendous accomplishment, the researchers write, because “there is an unmet need for novel classes of antibiotics, cancer drugs, antivirals and more.”
Explainable AI and the Secrets of Drug Discovery
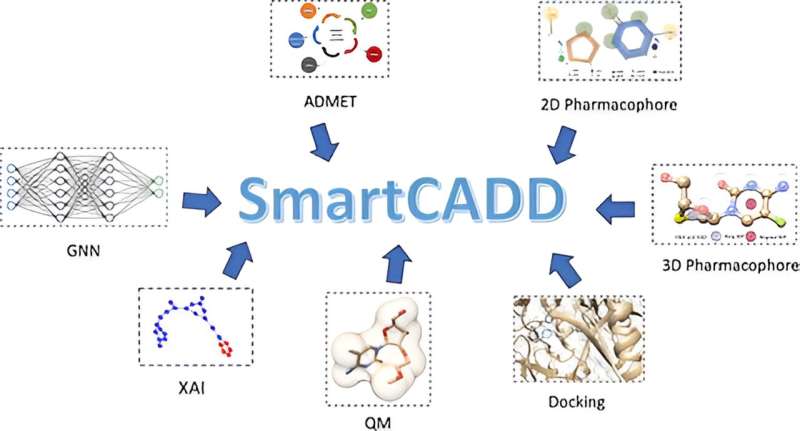
Among the many challenges that AI presents in scientific research is its use in black-box approaches, as well as concerns about the quality of data used for training, both of which are addressed in SmartCADD. This utilizes smoking deep learning models, filters proscribes, and explainable AI to mine chemical compound databases for potential drug leads.
The SmartCADD framework consists of two primary components: the Pipeline Interface that ingests data and executes filters; and the Filter Interface which specifies how each filter should function. These custom filters can aid everything from the development of predictors for drug-like behaviour and in-vivo activity, the depiction of structures by both 2D and 3D representations of a molecule to more recent ‘AI’ models that explain themselves. While researchers are always asking the platform for a higher level of transparency and those features are certainly part of what sets SmartCADD back, it is certainly hands down one of the most user-friendly options in implementation once you begin to use these features in your drug discovery workflow.
It Takes a Village: Interdisciplinary Collaborations Revolutionize HIV Drug Discover
To highlight the abilities of SmartCADD, the researchers performed three case studies covering drugs to treat HIV. The platform used data from the MoleculeNet library to build and scan an 800 million-compound database, identifying 10 million that could potentially be HIV drugs. It used it to filter out the compounds according their similarities with some of already developed HIV drugs.
SmartCADD is the direct results of an interdisciplinary collaboration between SMU’s chemistry and computer science departments. Drug discovery, for example, depends on a community to be truly successful,” said Ayesh Madushanka, postdoctoral research fellow in the Department of Chemistry. If it was left to the Chemistry department, I am pretty sure this would not have been the final product. Learning with different disciplines leads to innovative ideas on the same topic, which further shapes it better. The platform has to a great extent distinguished potential outcomes in the future and its makers are now forthcoming with curiosity on broadening the capabilities of this technological AI/quantum mechanics mechanism so that it could cater a broader outlook within industries such as drug discovery pipelines.
