Researchers have developed a novel synthesis method that significantly lowers the sintering temperature required for electrolyte densification in high-efficiency protonic ceramic cells. This breakthrough addresses the key challenge of high production costs and performance degradation in existing solid oxide cells, paving the way for the commercialization of next-generation fuel cells and electrolyzers.
Unlocking the Potential of Protonic Ceramic Cells
Existing solid oxide cells (SOCs) can generate electricity in fuel cell mode and produce hydrogen in electrolysis mode, operating at high temperatures above 600°C. This offers higher power conversion efficiency compared to other fuel cells. However, the high production cost and performance degradation due to thermal deterioration have been major obstacles to their widespread adoption.
Enter protonic ceramic cells (PCCs), the next-generation energy conversion devices that transport protons (hydrogen ions) instead of oxygen ions. PCCs boast higher ionic conductivity, enabling greater efficiency. But to produce the electrolyte for PCCs, sintering at temperatures exceeding 1,500°C was previously required, leading to issues like component evaporation and precipitation that degrade the electrolyte’s ion-conducting properties – a significant barrier to the commercialization of PCCs.
Breakthrough Synthesis Method: Lowering Sintering Temperatures
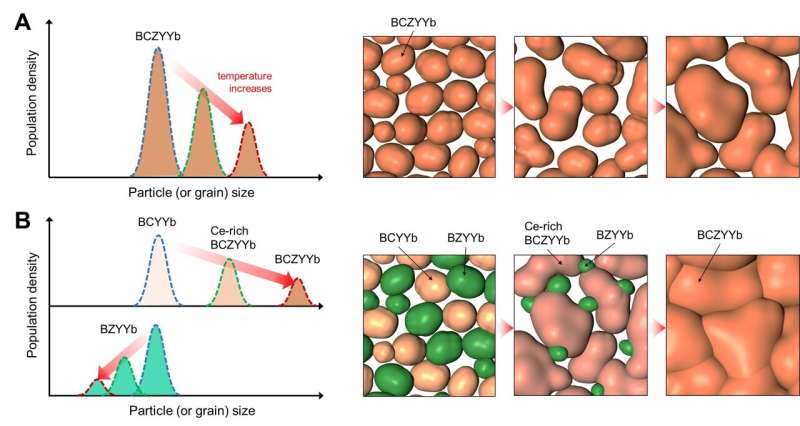
The research team has developed a groundbreaking new process for synthesizing electrolyte materials, overcoming the inherent sintering challenges. Typically, the electrolyte for proton ceramic cells is produced by sintering a powder composed of a single compound. However, when additives are used to lower the sintering temperature, residual additives can remain in the electrolyte, reducing the cell’s power density.
The researchers discovered that by synthesizing a powder containing two different compounds through low-temperature synthesis, a single compound with excellent sintering properties forms during the sintering process, accompanied by a reaction to a single phase. This innovative approach allows the sintering temperature to drop to a mere 1,400°C, without the need for any additives. The resulting proton ceramic electrolyte forms a dense membrane even at lower temperatures, significantly enhancing the electrochemical properties of the cell.
Unlocking Efficiency and Stability in Proton Ceramic Cells
When applied to actual proton ceramic cells, the new electrolyte synthesis process demonstrated superior proton conductance, achieving a power density of 950mW/cm2 at 600°C – approximately double that of existing cells. This breakthrough is expected to reduce process time and improve the thermal stability and performance of ceramic electrolytes, paving the way for the commercialization of proton ceramic cells.
The research team plans to apply this new process, which leverages the accelerated sintering between the two compounds, to the production of large-area cells, further advancing the commercialization of proton ceramic cells. This innovation holds the potential to enable efficient energy management through green hydrogen production via electrolysis and the utilization of waste heat from nuclear power plants for pink hydrogen production.
