A groundbreaking tool developed by researchers at the University of Missouri is using artificial intelligence to unravel the mysteries of protein complexes, paving the way for new treatments and transforming the future of medicine.
Scientists Crack Secret Code of Protein Interaction
Proteins form the backbone of life yet have proven themselves a tricky subject to understand for researchers. Cryo2Struct by Jack’s Lab: A groundbreaking tool for the interpretation of protein complexes using artificial intelligence
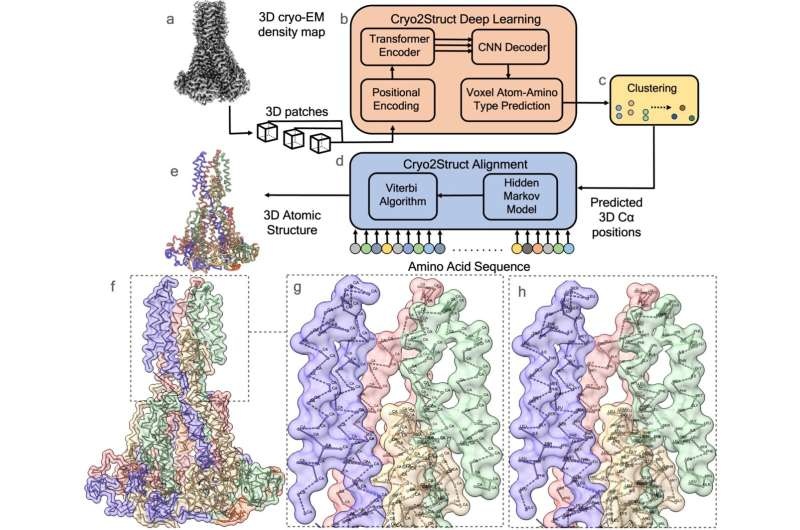
Historically, the construction of three-dimensional structures from large protein complexes has been a laborious and time-consuming manual task dominated by expert intervention in virtually every analytical stage. Now, Cryo2Struct breaks the mold–being the first method that fully automates this process to generate better structures than existing methods (3–5).
This is exactly what the service does; it can take a raw cryo-electron microscopy (cryo-EM) image and determine what its atomic structure should look like, without knowing in advance how that structure should be constrained. Our novel method gives scientists the tools necessary to identify and create a full 3D structure of protein complexes, as well as offers key revelations into their mechanisms.
Exploiting Protein Paradigms
One of the themes in basic biology is understanding protein interactions in the body, which are how diseases take a hold and grow; this kind of biological research tells scientists exactly how they will proceed to develop drugs for treatment. Here, the work of Cheng and Cryo2Struct are a game-changer.
With this 3D structure of protein complex in place the researchers can develop drugs to correct the aberrant function and help this very important protein work as it should. It broadens the scope for a range of diseases, including cancer, to be treated with new and better targeted therapies.
Cheng and his team also has been working on an alternative AI method called diffusion model to help scientists develop small molecules, for example drug compounds, find how & where those drugs would bind to a protein. Ideally, the technology could be used both to enhance existing drugs and develop more potent one s specific needs of patients.
Conclusion
Cheng and his team at the University of Missouri have just released their new reports detailing a forthcoming major shakeup in the realm of protein research as well as the further development of medicinal Apigenin (APG) treatment. Leveraging artificial intelligence, they developed a tool that can disentangle the intricacies of protein structures and interactions to enable the discovery of drugs against many diseases. This is a collaboration between specialties and displays the inherent power in interdisciplinary input into improving care, especially when assisted by novel technology.
