In a groundbreaking development, scientists at the Ningbo Institute of Materials Technology and Engineering (NIMTE) of the Chinese Academy of Sciences have discovered a novel anti-corrosion anode that enables stable seawater electrolysis for over 10,000 hours. This remarkable achievement paves the way for the commercialization of seawater electrolysis, a cost-effective and sustainable approach to hydrogen production. The team’s innovative solution, published in the prestigious journal Advanced Materials, could significantly impact the future of renewable energy and water conservation.
Beating the corrosion barrier
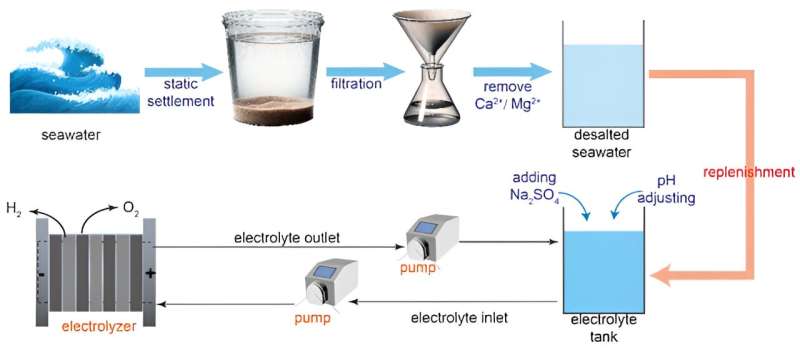
Therefore, seawater electrolysis provides a valuable alternative renewable energy pathway for hydrogen production that does not deplete freshwater resources. The biggest obstacles have been the very high chloride ion concentrations in sea water, these induced quite unbearable anode corrosion which completely blocks the possibility of using this technology on a large scale.
Now, scientists from the NIMTE have come up with a solution for this. They have creatively reported on catalyst design by doping the NiFe-LDH (NF-LDH) catalysts with Ba2+ to create NiFeBa-LDH (NFB-LDH) catalysts in a one-step hydrothermal manner. The first advantage of the NFB-LDH catalyst is the chemical fixation of free SO42− by atomically dispersed Ba2+, in situ forming a high-density SO42− layer to substantially suppress corrosive Cl- ion adsorption and protect the anode from corrosion.
Improved Stability and Performance, like Never Before
Both in alkaline seawater and alkaline saline electrolytes, the NFB-LDH anode after 10,000-hour operation at a constant current density of 400 mA cm−2 exhibits consistent performance. Such stability performance has never been shown before, representing a breakthrough step towards commercialization of seawater electrolysis technology.
The NFB-LDH anodes have higher stability and activity than commercial Ni under the simulated industrial condition. While the mitigation of corrosion is a major advancement, it benefits much more than just solving the corrosion issue in seawater electrolysis; it ultimately improves its overall efficiency and practicability. The researchers’ unique method offers the promise of transforming hydrogen production from seawater into a more commonly available and sustainable solution for renewable energy.
While this improvement takes us closer to using seawater as affordable hydrogen feedstock, the total electrolysis cell voltage is still higher than for pure water due to a slightly optimized hydroxide mobility
Thus, the new NFB-LDH with excellent anti-corrosion performance to serve as the superior anode material is a promising direction for energy-saving in aqueous alkali metal ion batteries. A technology that overcomes the historic barriers to seawater electrolysis and enables novel pathways for hydrogen production and storage while benefiting freshwater resources.
And the implications of this achievement are much broader than just energy. It could also have dramatic implications for water conservation and our energy systems’ impacts on environmental sustainability. Given the global challenges of climate change and water scarcity, discoveries such as the NIMTE researchers’ nanovesicles offer new hope for a more sustainable future.
Still more research and development will be needed to take the technology to scale and improve its performance, but the NFB-LDH anode is a prime example of the potential of cooperation and discovery in science. This breakthrough is therefore an essential milestone in the journey towards a sustainable tomorrow — showing that together, with continuous gains in knowledge and unwavering dedication to making our world greener, we can overcome the obstacles faced.
