In an effort to produce more sustainable, low-cost electrical energy, scientists at Johannes Gutenberg University Mainz (JGU) in Germany have enhanced the effectiveness and stability of water-splitting catalysts through a ground breaking optimization approach that pioneered new routes to designing advanced materials. The integration of high-valence molybdenum (Mo) into NiFeOOH results in such striking enhancements in catalytic activity and stability that now it can be considered a viable approach to commercialization for renewable energy technologies.
Unleashing the Power of Bimetallic Synergies
As a significant half-reaction of water-splitting for clean hydrogen fuel, the oxygen evolution reaction (OER) is pivotal but remains challenging. This has made noble metal-based catalysts like RuO2 and IrO2 the de facto choice, but their high cost and scarcity have inspired the quest for cheaper non-precious metal substitutes.
So is NiFeOOH, an OER catalyst that has come up as one of the best OER catalysts — cost-effective, highly active.Node (2), ID: 1450341 Nevertheless, getting it up to the performance level required for industry is a major challenge. It is here that the inventive approach utilized by researchers at Xi’an Jiaotong University becomes so critical.
They focused on modifying the electronic properties of NiFeOOH to promote its electrical conductivity via introducing high-valence molybdenum (Mo) cations into the structure; then regarded Xiangjiu Guan as provided the guidance led this project. The ultimate goal of this strategic doping was to lower the energy barrier for OER and also improve these catalysts operational stability.
Uncovering the Catalyst’s Secrets
The researchers used a variety of methods — from empirical experiments to theoretical simulations — to get at these complex interactions between Mo, Ni, and Fe in the doped catalyst.
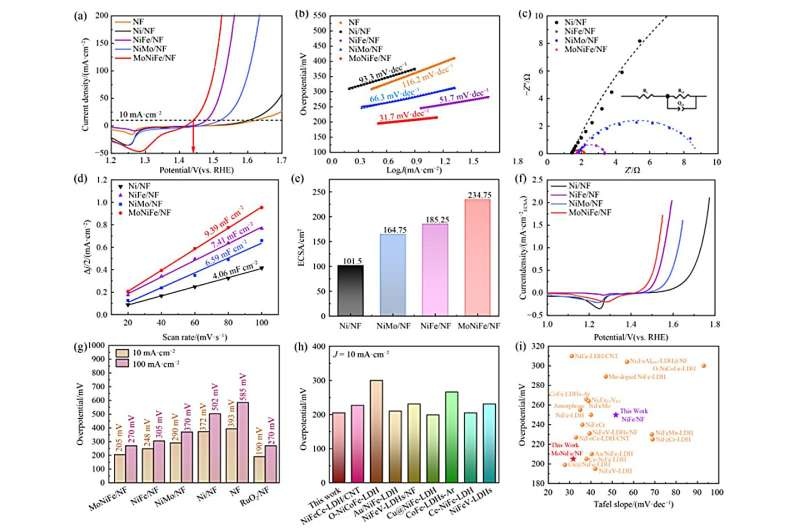
And the results were truly mind-boggling. Discharging a much lower overpotential of 205 mV at 10 mA/cm² (Fig. 4c).They also had better catalytic activity reflected with the Tafel plot and obtained a slope of only 31.7mV/dec, which is significantly smaller than other samples. In addition, it continued to function well in this way for 170 hours and finally retired due to the causes beyond its control.
The valence states of Ni and Fe are modified through the Mo-doping process as shown in another study which shifts the d-band center of bimetallic active sites. This change is important for the conversion to an active γ-NiFeOOH phase16,18 used for fast OER performance.
To reinforce their conclusions, the team performed density functional theory (DFT) calculators to pinpoint some of the most promising OER pathways and Mo’s effect on catalytic levels. The simultaneous of the empirical and theoretical studies provides a detailed view to explain what happens within the catalyst.
Conclusion
The work marks a breakthrough in providing an efficient and scalable solution for producing high-performance, non-precious metal electrocatalysts for the OER. The excellent features of this Mo-doped NiFeOOH catalyst—low overpotential, high reaction kinetics and long-term stability—would make it a strong candidate for practical applications in water electrolysis and other renewable energy technologies. Our work not only highlights the bimetallic synergy that can play a key role in catalyst design but, and more importantly, it enriches our understanding of electrocatalysis at the fundamental level, something canting towards further innovations in this paramount area.
