Researchers have uncovered a previously unknown involvement of sodium transport in the mitochondrial process of energy production, shedding new light on the fundamental mechanisms of cellular metabolism and opening up possibilities for tackling neurodegenerative diseases.
The Crucial Role of Sodium in Cell Energetics
Photo: Centre of hospitable services In the study, headed by a group of scientists from the National center investigation Cardiovascular (CNIC), discovered an essential role AffiliateAdvertTranslator in the creation of energy to cells is played by sodium.
Until the 1990s, most scientists believed that Nobel laureate Peter Mitchell’s chemiosmotic hypothesis was sufficient to explain how mitochondria make all their ATP currency cash: A proton gradient across the inner mitochondrial membrane did it all.
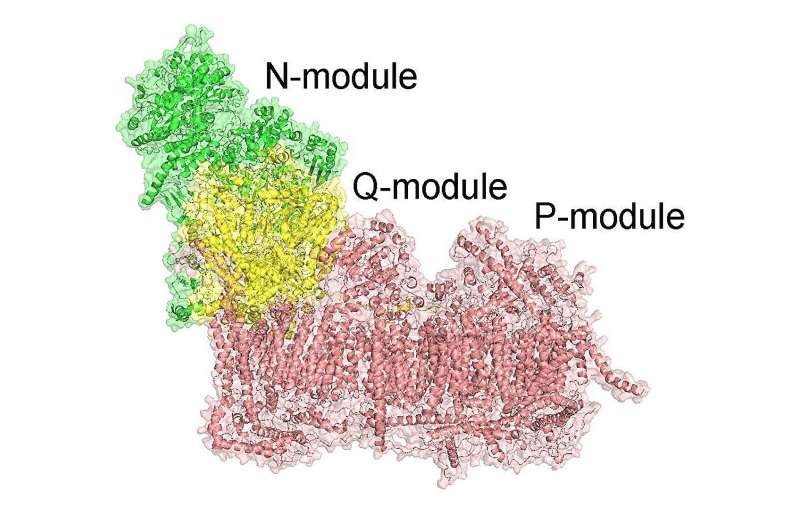
The new findings also suggest that the transport of sodium ions, which had been previously overlooked in this process, is involved as well. That’s why the research team used such diverse genetic models and mutants to show how mitochondria exchange sodium ions for protons via mitochondrial complex I which is the first enzyme in our electron transport chain, thereby, it makes a huge amount of sodium gradient which contributes half of our total membrane potential that is absolutely needed to make ATP.
The Solution To Leber’s Hereditary Optic Neuropathy
This activity of sodium transport by complex I also explains the molecular basis for the neurodegerative disease Leber’s hereditary optic neuropathy (LHON).
LHON is the leading mitochondrial genetic disease among all nations, caused by mutations in mitochondrial DNA. This new study demonstrates that the cause of hereditary optic neuropathy in LHON is a specific defect in sodium and proton translocation by complex I.
The sodium-proton transport activity was lost when researchers removed complex I in mice, but not with removal of the other complexes, they found. This indicates that the ability to transport sodium and protons directly depends upon the presence of complex I, providing a mechanism through which LHON can result.
These results suggest novel pathways for targeting sodium transport defects in mitochondria in the development of therapies to treat LHON and possibly other neurodegenerative conditions.
Conclusion
This finding that sodium transport is essential for mitochondrial bioenergetics, and therefore cellular metabolism and the pathogenesis of some neurodegenerative diseases marks a quantum leap in our understanding of these issues. The identification of this new role of mitochondrial complex I reveals a basic feature in cellular bioenergetics and provides opportunity to develop much needed disease-specific therapies for conditions like Leber’s hereditary optic neuropathy. This study is a milestone on the road to fully comprehending the complexity of biological systems and underscores that further exploration and collaboration are key to making such strides.
