Researchers have developed a novel method to rapidly synthesize highly stable gold nanoparticles (AuNPs) using a combination of citric acid, dihydrolipoic acid (DHLA), and DHLA-alanine under UV light. These AuNPs exhibit remarkable stability against high salt concentrations and temperature changes, making them promising for various biomedical and catalytic applications. The study provides valuable insights into the efficient production of customizable gold nanoparticles with enhanced properties. Gold nanoparticles, dihydrolipoic acid, and photoreduction are some of the key concepts explored in this research.
Harnessing the Power of Light for Nanoparticle Synthesis
Gold nanoparticles (AuNPs) have garnered significant attention in the scientific community due to their unique properties and diverse applications in fields such as catalysis, and sensing. Conventional methods for synthesizing AuNPs often involve thermal reduction, which can lead to undesirable aggregation and instability. To address these limitations, researchers have explored alternative approaches, including the use of photoreduction, where light energy is utilized to drive the reduction of gold ions and the formation of stable AuNPs.
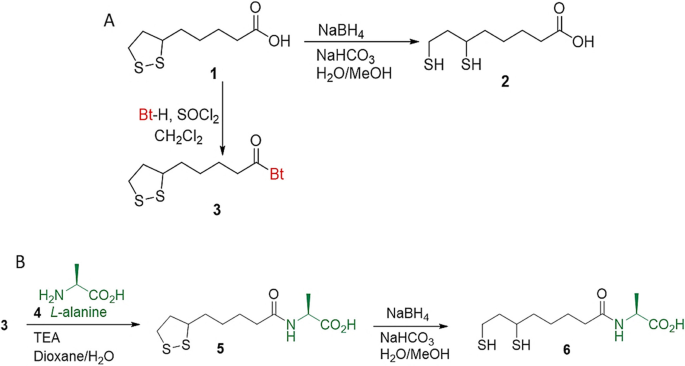
Rapid and Stable AuNP Synthesis with DHLA and Alanine
In this study, the researchers developed a novel method for the rapid and stable synthesis of AuNPs using a combination of citric acid, dihydrolipoic acid (DHLA), and DHLA-alanine (DHLA-Ala) under UV light irradiation. While citric acid served as a reducing agent to facilitate the reduction of gold ions (Au3+) to gold nanoparticles (Au0), DHLA and DHLA-Ala acted as strong stabilizing agents, binding to the surface of the AuNPs and preventing their aggregation.
The researchers found that the DHLA-Ala@AuNPs and DHLA@AuNPs were formed within just 10 minutes under UV light at room temperature, a significantly faster process compared to the traditional thermal reduction methods. Importantly, these AuNPs exhibited remarkable stability, maintaining their colloidal form and optical properties even in the presence of high salt concentrations and at different storage temperatures.

Unraveling the Mechanism of Rapid and Stable AuNP Formation
The researchers propose the following mechanism for the rapid and stable formation of AuNPs:
1. Citric acid binds to the gold ions (Au3+) and reduces them to gold nanoparticles (Au0) through an electron transfer process, facilitated by the UV light irradiation.
2. As the photoreduction reaction progresses, the concentrations of citric acid and gold ions decrease.
3. The reduced DHLA and DHLA-Ala molecules then rapidly bind to the surface of the gold nanoparticles through their thiol (-SH) groups, forming a protective layer and preventing aggregation.
4. The adsorbed citrate anions and DHLA/DHLA-Ala molecules on the AuNP surface provide electrostatic stabilization, further enhancing the stability of the nanoparticles.

Catalytic Activity and Potential Applications
The researchers also investigated the catalytic activity of the synthesized DHLA-Ala@AuNPs and DHLA@AuNPs by studying their ability to catalyze the reduction of 4-nitrophenol to 4-aminophenol, a reaction that is commonly used as a model system for evaluating the catalytic performance of nanoparticles. The results showed that both types of AuNPs were effective catalysts, with DHLA@AuNPs exhibiting a faster catalytic rate compared to DHLA-Ala@AuNPs.
The enhanced stability and catalytic activity of these AuNPs make them promising candidates for a wide range of applications, including:
1. Catalysis: The efficient catalytic performance of the AuNPs could be leveraged in various chemical reactions and environmental remediation processes.
3. Sensing: The unique optical properties of the AuNPs can be exploited for the development of sensitive and selective sensing platforms.

Advancing the Field of Nanoparticle Synthesis
This study represents a significant advancement in the field of nanoparticle synthesis, demonstrating a novel and efficient approach to producing highly stable gold nanoparticles. The combination of citric acid, DHLA, and DHLA-alanine, coupled with the use of UV light irradiation, allows for the rapid and one-step formation of AuNPs with exceptional stability against environmental factors.
The researchers’ findings contribute to a better understanding of the mechanisms underlying the photoreduction of gold ions and the stabilization of the resulting nanoparticles. This knowledge can be leveraged to further optimize the synthesis of various metallic nanoparticles, opening up new avenues for their application in diverse fields, from biomedicine to catalysis and beyond.
As the scientific community continues to explore the vast potential of nanomaterials, studies like this one highlight the importance of developing innovative synthesis methods that can unlock the unique properties and functionalities of these tiny yet powerful structures.
Author credit: This article is based on research by Nimet Temur, Seyma Dadi, Mustafa Nisari, Neslihan Ucuncuoglu, Ilker Avan, Ismail Ocsoy.
For More Related Articles Click Here
