Researchers have developed a groundbreaking technique using DNA origami and fluorescent probes to precisely control the release of molecular cargo, paving the way for advancements in targeted therapies and cellular research.
Detecting and Manipulating Lipid Vesicles
The emerging notion in nanotechnology is creating smart systems that sense molecular signals. One great example is the new tech-industry DNA origami technique in which DNA can be coded to fabricate custom-designed nanoscale building blocks of any shape.
Scientists at LMU Munich led by the chemist Philip Tinnefeld now present new results in the journal Angewandte Chemie, which indicate a strategy for selective recognition and controlled release of molecules with lipid vesicles using DNA origami and fluorescent probes.
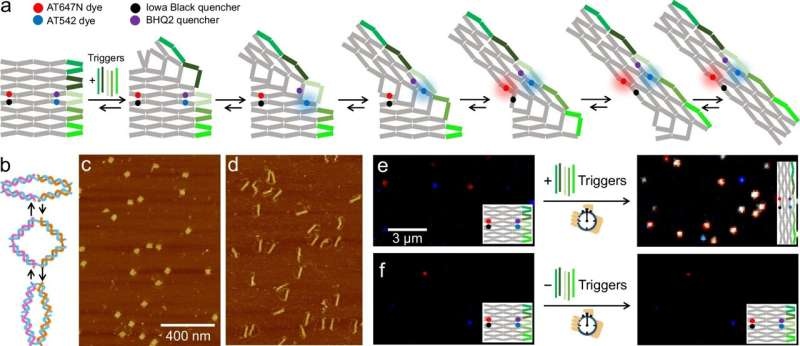
The device functions through single-molecule Fluorescence Resonance Energy Transfer (smFRET), which involves measuring the distance between two fluorescing molecules. The DNA origami structure which has a single-stranded DNA labeled by a fluorescent dye at the tip of the probed strand. The fluorescence signal changes upon binding of this DNA strand to a lipid vesicle; the conformation change results in an increased/decreased distance from a dye at the end of the DNA strand to another nearby fluorescent molecule through cellular origami structure. This provides a way to detect vesicles, then in a follow-up, the system carries molecules where the sensing strand acts as a molecular cargo that can be transferred into the vesicle.
Controlling Molecular Cargo Transfer
Building upon their innovation, the researchers further engineered the system to control with high spatial precision when the molecular cargo is transferred. Since lipid vesicles are so central in cellular processes from molecular transport to signal transmission, the ability to perform such detection and manipulation on these membrane bound objects offer possibilities for biotechnological applications including drug targeting.
Such an approach revealed in this study might be generalized for loading lipid nanoparticles with uniform number of molecules relevant to a wide range of applications including vaccines. The possibilities of the new system are no less interesting for biological research, where it could be employed to gain a better understanding and develop interventions against cellular processes at the molecular level. “Our system also offers promising approaches for biological applications,” adds Tinnefeld.
In a separate study published in Nature Communications, Tinnefeld and colleagues led by Yonggang Ke from Emory University introduce a DNA origami structure that allows them to monitor the stochastic morphing of a stepwise allosteric conformational change upon moderator-strand binding. Now, the scientists succeeded in performing such studies at the molecular level and showing how the reaction steps can be temporally controlled by means of FRET probes using photoswitchable PerCP. They also demonstrated that a DNA cargo can be specifically released during this process, paving the way for controlled reaction cascades.
Conclusion
Research groups led by Philip Tinnefeld in Munich and Yonggang Ke at Emory University have now exploited the methods for exact positioning of fluorescent probes, and use DNA origami as a tool to accurately control the release of molecular cargo. It is a game-changing method with enormous implications for clinical targeted therapies, vaccine development and cellular processes as viewed through the landscape of the molecular vortex. To control cells with real precision, the researchers believe that future work has to rely on intelligent strategies in engineering dynamic molecular machines capable of reacting only when specific non-invasive molecular signals are detected.
