Groundbreaking research from Osaka University has unveiled a new mechanism crucial for the initiation of autophagy, a process cells use to eliminate unneeded or damaged components. This discovery could have far-reaching implications for understanding and treating various diseases, including cancer and neurodegenerative disorders.
Involvement of Palmitoylation in the Regulation
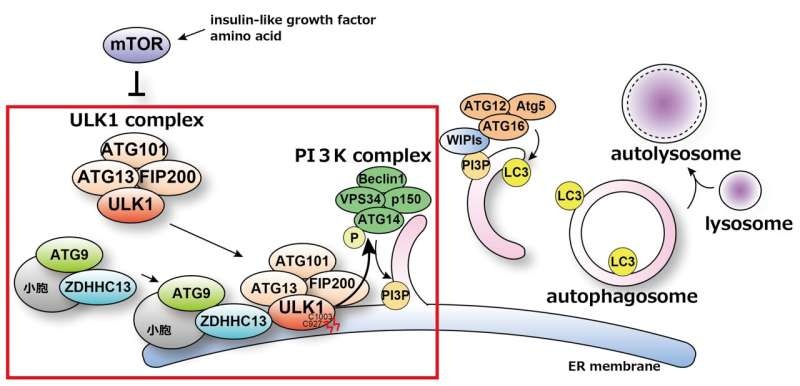
In a major breakthrough toward understanding the initiation of autophagy, a research team led by Osaka University (Japan) found structures that essentially function as “the on-switch” for mouse autophagy. They found that one of those proteins, ULK1, requires palmitoylation which is acylation particularly of a cysteine residue and that this process is essential to mediates stress-induced autophagy.
Palmitoylation is the post-translational modification with a reversible addition of palmitate, which is a 16-carbon saturated fatty acid, on proteins that regulates protein localization, stability and function [5]. The scientists know that palmitoylation enzyme ZDHHC13 is responsible for modifying ULK1 like this. This palmitoylation primes to the induction of a cascade of reactions that result in the generation of autophagosomes, which are double-membraned vesicles responsible for engulfing cellular materials for degradation upon initiation of autophagy.
Mutations in the gene ZDHHC13 result in several diseases, notably Huntington… This indicates a potential role for the failure of palmitoylation-dependent autophagic initiation in pathologies. How autophagy could be regulated by this mechanism might also open up new avenues to target these diseases.
The Pivotal Role of Autophagy
Autophagy is one of the basic cellular processes that has received attention due to its involvement in aging, and lifespan regulation. As an example, cells participating in the process of autophagy isolate and then consume proteins or organelles in order to reuse their parts and produce energy.
It is extremely important for these pathologies to be stopped, but it is not necessary in order for normal cellular functions to work properly. Autophagy disruptions have been suggested to contribute to cancer, as well as a number of neurodegenerative diseases.
The research team’s discovery reveals the molecular mechanism that triggers autophagy, an essential advance in understanding how this process is regulated to treat autophagic deficiencies, and diseases related to autophagy such as Crohn’s disease. Their discovery of how palmitoylation determines the cellular location and activity of the ULK1 complex defines a novel target for future therapeutic approaches to diseases.
Conclusion
For the first time, the Osaka University group has discovered a mechanism that determines the start-up of autophagy and thus uncovered crucial insights affecting human health. By revealing the critical contribution of palmitoylation to ULK1 complex localization and activation, the study sheds light on control mechanisms that govern the core process responsible for cell survival. Such insight into these molecular mechanisms would be fundamental to elucidating the pathogenesis of autophagy-related diseases and inspire novel therapeutic targets spanning from cancer to neurodegeneration. The findings come as key advances in understanding how autophagy, an important cellular recycling process that keeps our bodies healthy and is critical for maintaining overall health including lifespan, opposes the progression of diseases.
