Researchers have discovered a novel mechanism that determines the spiral shape of the photosynthetic bacterium Rhodospirillum rubrum. By studying the arrangement of certain proteins in the outer membrane, they found that a specific lipoprotein, called PapS, plays a crucial role in the curvature of the cell wall, leading to a distinct spiral shape. This finding could provide insights into how cell shape affects the way bacteria colonize their environment, interact with other organisms, and even cause disease. Bacteria come in a wide variety of shapes, and understanding the factors that influence their morphology is key to understanding their biology and potential applications.
The Surprising Role of Outer Membrane Proteins in Bacterial Cell Shaping
Bacteria come in many forms — scientists have long been intrigued by their array of shapes and sizes, from the most familiar straight rod (an E. coli bacterium) to those with a curve or spiral. The shapes of these cells are more than just a pretty face; they play an important role in bacterial biofilms, mobility through viscous environments, and— perhaps most sinisterly—in facilitating disease.
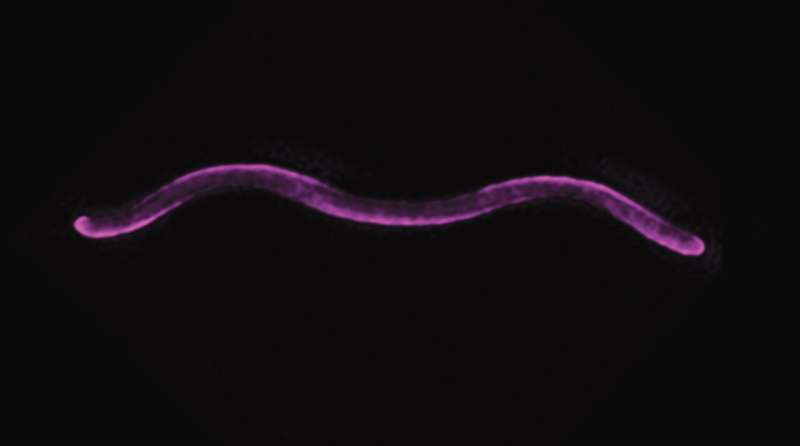
A team led by Martin Thanbichler, Max Planck Fellow and Professor at the University of Marburg in Germany, has now discovered a new mechanism that specifies the helical morphology of Rhodospirillum rubrum, a spiral-shaped photosynthetic bacterium. In the environment, it is ubiquitous and its biotechnological potential is tremendous since it can grow on carbon monoxide, fix nitrogen as well as produce hydrogen and building blocks for bioplastics.
The Helical Arrangement of Outer Membrane Proteins and Their Impact on Cell Cu
When they looked at the purple bacterium Rhodospirillum rubrum, however, they found that instead of forming the sporelike outer membrane vesicles typical of hollow polyhedral shapes or pillow shapes during the stationary phase in Escherichia coli and P. aeruginosa samples, two porin proteins that normally mediate nutrient exchange across the outer membrane adopted helical windings along the outside of the cell. These porins are tethered by another protein, the lipoprotein which is itself tightly associated with the cell wall.
In doing so, the researchers were able to make the cells completely straight by preventing PapS from binding to the porins, underscoring the importance of this lipoprotein in shaping the spiral form of Borrelia. Through these other studies, the team found that Porin-PapS’s structure is more akin to a “molecular cage” for machinery responsible for the longitudinal growth of the cell. This unequal growth over the cell body is the basis of the characteristic spiral shape of Rhodospirillum rubrum.
Implications for Understanding Bacterial Ecology and Potential Applications
This study proposes a new aspect of bacterial cell physiology, which may provide an important clue to elucidate the behavior and interaction of bacteria in natural habitats. Curvature and spiral shapes are known to affect the ability of a bacterium to colonize surfaces, traverse viscous media, and establish symbiotic relationships with other organisms.
This discovery paves the way to genetically-engineer two morphological variants of Rhodospirillum rubrum, and gives insight into how cell shape influences R. rubrum’s ecological niche (including:: symbiosis with plants as well as virulence), was highlighted by Henry and co-workers in FEMS Microbiology Ecology. Understanding the processes that underlie cell size control in that type of either unipolarly or bipolarly growth rapidly could have important consequences for therapeutic approaches in bacteria from absence and novel functions to curved or helical morphology if the organism is not found as mentioned here; or even for biotechnological uses.
