Research performed at CNIC, investigates the role of sodium transport in generating energy in a new light and have significant implications for understanding neurodegenerative diseases such as Leber’s hereditary optic neuropathy (LHON).
Sodium’s Surprising Symphony
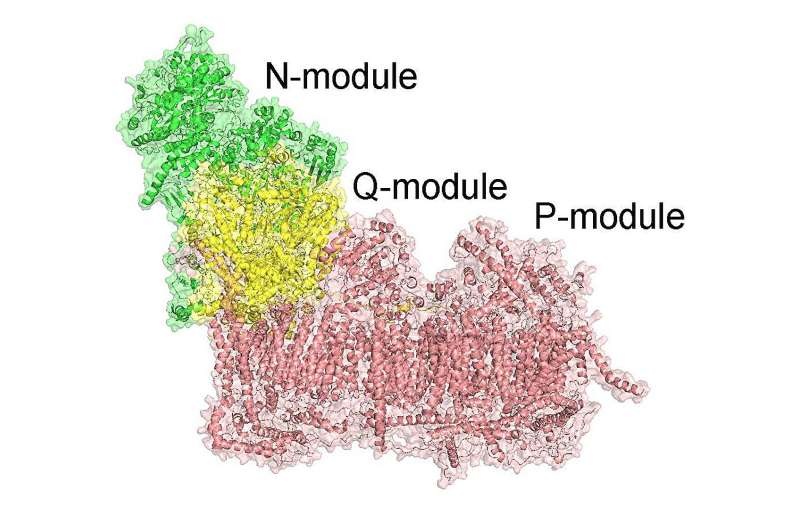
This is one of the remarkable findings of a study published today in Science, led by CNIC’s Dr. José Antonio Enríquez and conducted by his team, showing that respiratory complex I –a key enzyme in the mitochondrial electron transport chain– constitutes a novel secondary sodium pump with bioenergetic functions This transportation of sodium is essential for optimal production of cellular energy by a process called oxidative phosphorylation.
ATP synthesis, which is essential for the functioning of any cell and thus represents the main source of energy in a living organism, corresponds to the chemiosmotic hypothesis, it is postulated that an electrochemical gradient of protons generated over mitochondrial inner membrane leads to each production. But the deep spaces are a different matter, and as it turns out, sodium ions also play an important role in this process– something that wasn’t really considered until now.
Solving the Enigma of LHON
This reveals a new and important type of sodium transport associated with pathogenic disease: the sodium-based mitochondrial calcium efflux carrier and provides insight to the molecular basis for Leber’s hereditary optic neuropathy, identified as resulting from primary LHON mutations. LHON,first reported in 1988,is one of the most common mitochondrial genetic disorders,responsible for mutations within mitochondrial DNA.
Now, the research team has defined this as the defect that leads to all manifestations of hereditary optic neuropathy in LHON: specific sodium and proton translocation by complex I.
Using an assortment of mutants and genetic models, the researchers found that mitochondrial complex I swaps sodium ions for protons to create a parallel gradient of sodium ions. The sodium gradient generated by mitochondria and eventually re-established after Na+/Ca2+ counter transports in exchange for inward Ca2+ onto mitochondrial matrix plays an important role in cellular physiopathology including energetics, despite the sodium component has at best been considered to be a poor substitute for protons [4, 9] and widely disregarded (probably unfairly) as physiologically insignificant or harmful; it may form near half of the inner membrane potential with crucial relevance to ATP production!!
Conclusion
Their crucial role in energy generation by mitochondria has until now been unrecognized, as well the possibility that disorders of sodium transport might be targets for therapy in neurodegenerative diseases such as LHON. The researchers speculate that sodium-proton transport deficiencies could also be involved in other neurodegenerative diseases, including Parkinson’s disease (complex I involvement is evidenced there). This has the potential to completely change how we look at ATP and how it is produced in cellular systems, with impacts on Modern understandings of metabolic diseases –Alzheimer, Parkinsons that can lead to more specific therapies for these conditions.
