Discover how a cutting-edge combination of silver nanoparticles and a new sensing method are revolutionizing the fight against antibiotic-resistant biofilms, a critical challenge in modern medicine.
Nanoparticles: The Hidden Allies
The increasing threat of resilient biofilms has also highlighted the problem of antibiotic resistance in bacteria. standard antibiotics are often unsatisfactory, so medical personnel and scientists alike go to work trying to develop alternative options.
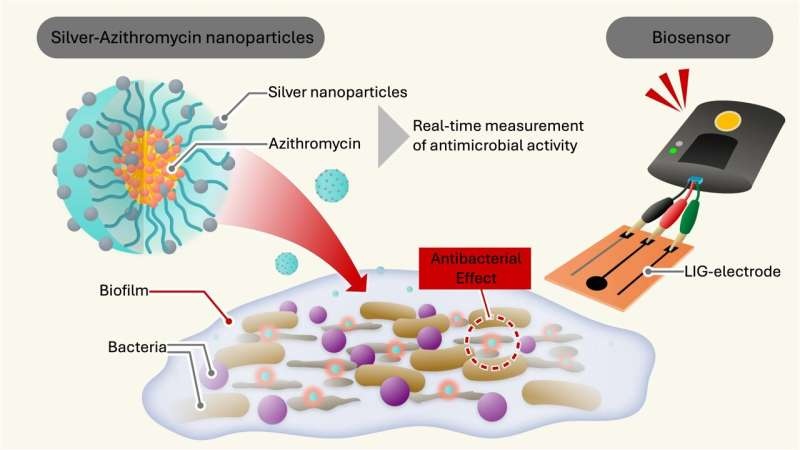
Here come silver nanoparticles. The trouble with biofilms is they are notoriously difficult to dislodge but these micromachines, tiny beasts that they are, have been engineered specifically to cut through the detritus and take it down. Through this technique, the researchers have engineered a nanoparticle that encases silver particles in an antibiotic azithromycin-carrying polymer shell that can penetrate and destabilize the biofilm’s protective coatings.
The double onslaught on the bacterial cells using silver ions and antibiotics has potentially game-changing implications for the treatment of resistant infections. This polymer shell keeps these nanoparticles stable and potent, rendering them a formidable weapon against superbugs.
A Sensor that Sees Through the Biofilm
Even though the nanoparticles look like a potential cure, the researchers realized that they must be able to test how well their treatment is working. Cursopty-Driven™ biofilm disruption assays are designed to solve this issue by allowing a rapid way to quantify the effect of disrupting agents on either growing or static biofilms in about 15 min — you even do not need instruments as traditionally for monitoring methods, like SEM or optical density measurements and/or specific dyes.
To address these challenges, the team went on to pioneer a type of sensing technology based on laser-induced graphene (LIG) electrodes. The bacteria cause problems by secreting biofilm-dissolving enzymes that can be picked up as an electrochemical response on the super-sensitive electrodes.
LIG sensor system enables rapid detection of the antimicrobial activity of the nanoparticles, in real-time, at high accuracy and low cost without any sample preparation or staining. Their method is a clean and scalable way to detect, control and track bacterial contamination and biofilms in the clinic which could lead to advances in areas such cancer screening and medical device coatings.
Conclusion
Resistance to antibiotics among some bacteria poses one of the biggest threats in modern medicine and using a LIG sensor-and-silver-nanoparticles combination provides a unique new tool to address this challenge. Taken together, the two-prong strategy to wipe out these persistent biofilms and the newly acquired capability of real-time monitoring of nanoparticle performance could help transform our stratagems in treating hospital-acquired infections as well as other deadly bacterial challenges. This collaborative, interdisciplinary research are crucial in battling the superbugs scourge that lies ahead of us.
