A new catalyst developed at Osaka Metropolitan University can directly convert waste plastics to diesel and gasoline analogs. This innovative solution will drive towards a sustainable future for an eco-friendly pathway in the chemical sector.
Tackling the Glycerol Surplus
Biodiesel production substantially increases glycerol generation as a byproduct, which has to be effectively used up. According to the researchers of Osaka Metropolitan University, they have found a solution for this problem.
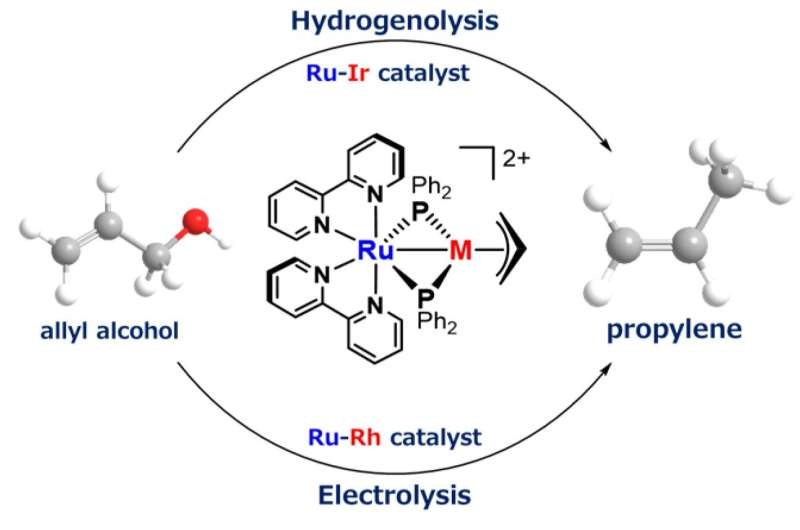
The new catalyst developed by them allows the selective conversion of a glycerol-derived compound, called allyl alcohol, to bio-based propylene. Propylene is a versatile chemical used to make many practical applications ranging from automotive bumpers, food containers and wood flooring. Historically, propylene was largely sourced from petroleum, a non-sustainable and environmentally-unfriendly process.
An international research team led by Associate Professor Shin Takemoto and Professor Hiroyuki Matsuzaka has developed a catalyst that can selectively and efficiently convert this glycerol byproduct into an important chemical, a step towards sustainability in the chemical industry.
The secret to conversion — being selective
Researchers have now solved this problem by coming up with a novel catalyst design that has enabled them to take the key step in the production process. The catalyst has a unique structure that makes it easy for it to bind two metals and separate them at the end of the process, using an innovative molecule called metalloligand.
An advantage of this new feature is that it promotes an efficient reaction, high selectivity and suppresses byproduct formation. Catalysis (electronic supplementary material, video S5), so that the oxygen-carbon bond of allyl alcohol is selectively cleaved–posing a radical improvement to both conventional and most existing bio-based methods to produce propylene.
By using renewable energy sources like hydrogen or electricity, the benefits of this approach are multiplied when it comes to the environment. Using these renewable energy sources, the researchers have at the same time found a method of production for environmentally sound chemical manufacturing.
Conclusion
This breakthrough by Osaka Metropolitan University researchers is huge for the chemical industry going forward. Thus, by converting a waste product of biodiesel production into a useful chemical they have shown potential for such sustainable and ecofriendly solutions. As the catalyst tackles glycerol byproduct surplus, it represents a greener alternative to established propylene production routes and thereby supports advancement toward circular and more environmentally sustainable chemical industry. With an eye on the global goal of carbon neutrality, such innovations are central to achieving a cleaner and healthier world.
