Discover how the unassuming hydrogen sulfide molecule plays a pivotal role in regulating iron uptake and antibiotic resistance in the notorious pathogen, Escherichia coli.
The Surprising Sidekick: Hydrogen Sulfide
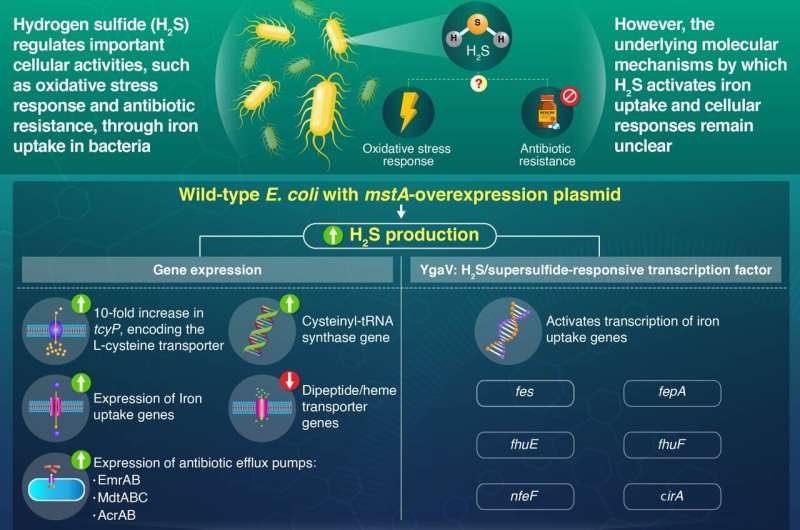
Although hydrogen sulfide (H2S) is also known as the smell of rotten eggs, this relatively unassuming chemical compound acts as an important signaling molecule in bacteria. Previous research has revealed a stunning capacity of this factor, to control several cellular processes such as antibiotic resistance and the oxidative stress response in pathogenic Escherichia coli.
The interesting aspect of H2S is that it can regulate iron uptake in bacteria. Higher levels of the metal reduce intracellular oxidation in a pathogenic bacterium, Vibrio cholerae by fueling synthesis of the amino acid, cysteine, which is part of glutathione, an antioxidant. Yet, the molecular basis for this cellular response to measurement of H2S in E. coli has not been defined — until now.
Unraveling the Mystery: E. Coli’s Iron Uptake Secrets
So a research team headed by Professor Shinji Masuo at Tokyo Institute of Technology wanted to investigate what role, if any, intracellular H2S might be playing in the uptake of iron by the notorious pathogen E. coli.
To address this, they combined two strategies: a genetically engineered wild-type (WT) E. coli strain with an exogenous overexpression of the mstA gene responsible for the production of H2S and productive solutions for sequestration. So that they could see what the increase in cell permeability via elevated H2S has on E. coli gene expression and cellular function.
Using then more sophisticated genetic sequencing methods and assays, the scientists investigated which molecular pathways behind iron acquisition respond to H2S availability. Their results, published in the prestigious mBio journal, provide important clues regarding this complex interaction.
To begin with, the team noticed that an overexpress of mstA directed in the WT strain produced high intracellular levels of H2S and potentiated antibiotic resistance — a worrying feature for a pathogen. This prompted them to delve more deeply into the hows.
Conclusion
Research conducted by the Tokyo Institute of Technology team has revealed that hydrogen sulfide regulates iron uptake in E. coli, thus providing new insights into how this toxic gas not only prevents oxidative stress but also acts as a signaling molecule in bacteria. In doing so, they unraveled the complex interplay of H2S, a protein designated YgaV that activates other genes and the expression of iron uptake genes — information that may lead to future strategies for breaking the resistance barrier in E. coli infections. In our fight against the increasing threat from antimicrobial resistance, this knowledge of how bacteria function is crucial in establishing new methods to reduce their effects on human health.
