Hydrogen-powered internal combustion engines hold immense promise in reducing carbon emissions, but they still emit nitrogen oxides, a harmful pollutant. Scientists at UC Riverside have discovered a cost-effective solution to significantly reduce this pollution by enhancing the efficiency of catalytic converters. Their innovative method using platinum-infused zeolites can convert nitrogen oxides into harmless nitrogen gas and water, paving the way for even cleaner hydrogen engines.
FUTURE BEHIND HYDROGEN ENGINES REDUCED EMISSIONS
Burning hydrogen with internal combustion engines is one possible solution in the fight against climate change. They have the power, but none of the earth-scorching carbon dioxide is given off in this process. Applications include heavy-duty vehicles like trucks and buses, as well as some off-road and agricultural equipment, and even emergency backup power generators which would be much cleaner than traditional diesel engines.
Yet, hydrogen engines are not free from emissions entirely. They can also emit nitrogen oxides as a byproduct of the high-temperature combustion process. Nitrogen oxides can combine with other substances in the air to produce ozone and issues such as fine particles, which are dangerous to lungs and can cause long-term health issues. Solving this dilemma is vital for unlocking the true power of hydrogen engines as we make the lifestyle towards a greener future.
Zeolites Come to The Aid of Catalytic Converters for their Best-ever Performance
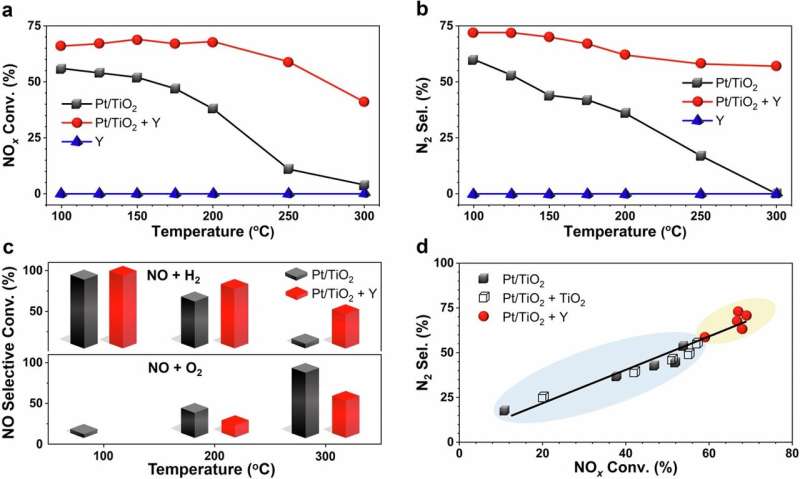
Now, researchers at the University of California, Riverside have discovered a cheap way to cut nitrogen oxide emissions from hydrogen engines by up to 90 percent. In a paper published in the journal Nature Communications, the researchers show how replacing a standard industrial coating material (CeO2) with Y zeolites can significantly improve catalytic converters used to combat emissions from gasoline and diesel engines.
Combining the best catalyst, platinum, in catalytic converters with Y zeolite dramatically improves nitrogen oxides and hydrogen reactions to convert them into harmless nitrogen gas and water vapor. The new catalyst converted nitrogen oxides into benign gases like nitrogen and oxygen four to five times more efficiently than the same one without zeolites did, at an engine temperature of only 250 degrees Celsius. However, as Global NCAP discovered with test at 0 deg C (32 deg F) — turbocharged for quick warm-up and fast odor reduction after starting the vehicle in tough cold climates — the system was most effective given those low temperatures mean available emission of pollution during engine startup.
Zeolite-Platinum catalyst has broader Versatility and Scalability
This new kilogram scale catalyst system opens the door to more than just hydrogen engines. The technology could also be used to help cut emissions from diesel engines with hydrogen injection systems — similar to the selective catalytic reduction systems fitted to many large trucks, the researchers note.
That catalyst system is so efficient basically because of what zeolites can do. These are inexpensive materials, each crystalline with a specific structure dominated by the elements silicon, aluminium and oxygen. The three-dimensional uniform pores and channels are uniquely advantageous in allowing greater surface area to increase the efficiency of pollutant break-down. The researchers developed a way of mixing platinum with the Y zeolite to create a system that traps most of the water formed in hydrogen combustion, thereby allowing hydrogen activation and improving nitrogen reduction efficiency.
Their work proves that this notion is not just limited to Y zeolite, and can be extended on other kinds of zeolite as well; this could offer an alternative method that is both adaptable and scalable for abating emissions from various types of internal combustion engines.
