Scientists at Chiba University created world-first visible-light-antenna ligand to meaningfully reduce the consumption of the expensive and scarce samarium for catalytic reactions in organic synthesis, thereby efficiently providing a tool to perform more cost-effective synthetic methodology in organic chemistry.
Harnessing the Power of Visible Light
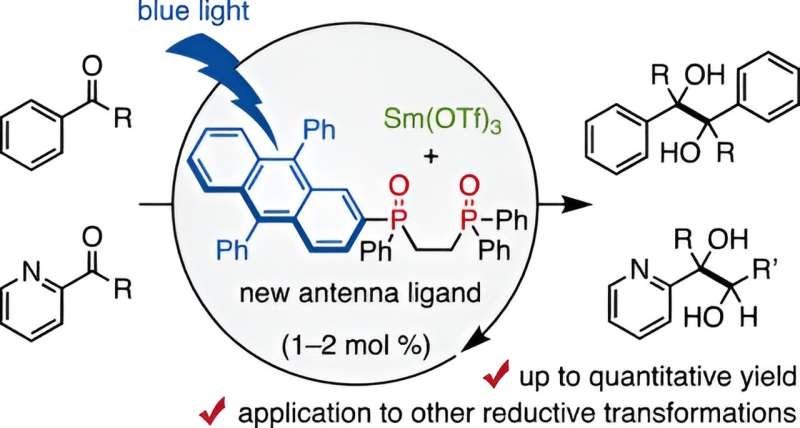
Particularly in the realm of organic chemistry, samarium (Sm) has been a go-to candidate [3] in the family of rare-earth metals because it is uniquely able to effect such very efficient single-electron transfer reductions. Unfortunately, conventional approaches using samarium iodide (SmI2) have been material-intensive and a cost prohibitive operations benefited from stoichiometric quantities of the metal. The problem which the research team led by Assistant Professor Takahito Kuribara of Chiba University has developed a new solution for is as follows. They developed 9,10-diphenyl anthracene (DPA)-substituted bidentate phosphine oxide ligands that bind trivalent samarium and enable the application of visible light to activate Sm in catalytic reductive transformations. This ‘visible-light antenna’ ligand cuts the required amount of samarium by orders of magnitude – only 1–2 mol% of the catalyst is now needed instead of the stoichiometric amounts usually required in reactions.
Unlocking New Possibilities in Organic Chemistry
For the first time, that team has crossed into a new frontier of organic chemistry. They obtained high yields of up to 98% in the pinacol coupling reactions of aldehydes and ketones, a common building block used in pharmaceutical industry, by using visible-light-antenna(3). Notably, the researchers were also able to facilitate these reactions even with weakly reactive organic reducing agents, e.g. amines where as before only strongly reactive reducing agents had been needed. This makes the process not only easier, but safer and better for the earth. In addition, the team found that a small amount of water helped with yields, He added: “Too much water can inhibit the reaction.” This shows that these chemical processes are very delicate, and require a balance and optimization of the controlled by selective formation of intermediate radicals.
Conclusion
This discovery is a major step towards expanding the capabilities of organic chemistry, according to the Chiba University research team. In this way, they have made it possible to carry out chemical reactions more efficiently and economically, especially in the field of pharmacy. This new way not only addresses the inadequacy of a lot of these processes in being truly sustainable, it opens up a whole host of molecular transformations that were previously either physically impossible or so expensive that their applications have been limited.
