A newly created process called mistargeting-specific molecule access to organelles (“MIMO”) has given the researchers at Stanford University a revolutionary way to guide proteins left awry within cells home, making it possible to fix broken marketing signals and offering new opportunities for treatments in many different diseases such as cancers, neurodegenerative diseases.
Balancing the Cellular Balance
Cells are well-organized structures with numbers of proteins which perform important functions. On the other hand, incorrect folding of proteins can have severe implications on health.
Protein mislocalization is only one possible outcome of this process (e.g., many diseases including cancers and neurodegenerative disorders are linked to the misplacement of proteins). In the specific case of some cancers, one protein that is supposed to keep track of what is going on with respect to DNA replication into the nucleus and far away from where it should be, allowing cancer to grow uncontrolled.
Now, a team of researchers led by Steven Banik, an assistant professor in chemistry at Stanford University, has devised a different approach. The scientists created molecules, which they call ‘Targeted Relocalization Activating Molecules’ (TRAMs), that prod the cell’s own shuttles to ferry out-of-place proteins back to where they belong in a cell.
A Cellular Jigsaw Puzzle
Cells are complex, busy places where proteins move around continuously and interact with many other molecules A protein, to work properly, must be where and when it is needed.
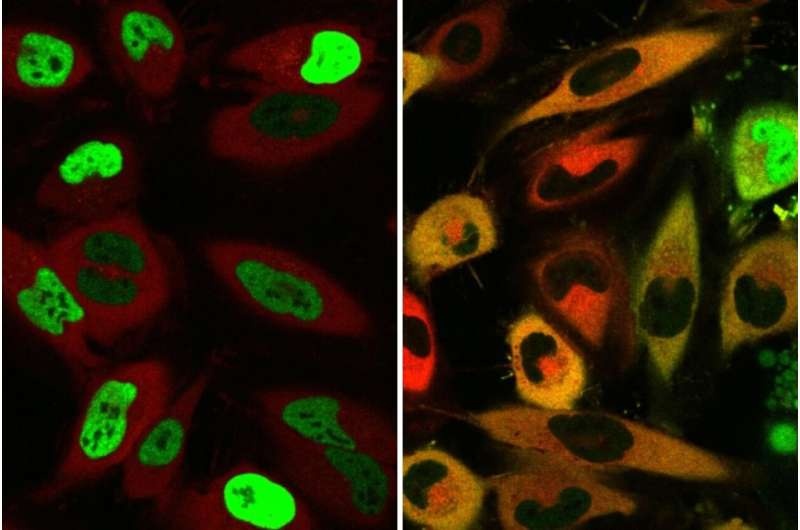
But a mutation associated with disease can upset that balance, redirecting proteins when those signals should not be sent. In ALS, one mutation results in the protein FUS exiting the nucleus and collecting in the cytoplasm, where it ultimately kills cells.
TRAMs created by the research team serve as a molecular shuttle, which move faulty proteins to the right place in the cell. It comes from the fact that TRAMs exploit a cell’s natural transport mechanisms, which can correct mislocalization or in simpler terms, the tendency of new proteins to end up someplace where they aren’t supposed to be raided by many disease-causing mutations.
Conclusion
This novel technique to repurpose misdirected proteins within cells is a significant advancement towards future disease therapy. Through stabilising the correct cellular balance, the researchers believe they will create an opportunity for treatments to target not only individual diseases such as cancers or neurodegenerative disorders. Such innovative work opens up a new dimension in the complex and surprisingly interconnected cellular world, promising to not just benefit patients but also expand our knowledge.
