Endosymbiosis, the phenomenon where one organism lives inside another, is a fascinating area of biological research. This study explores how bacteria can be injected into fungi to better understand the origins of this unique relationship. The findings shed light on the fragile nature of early endosymbiotic systems and the adaptations required for successful coexistence. Key insights include the initial decline in host fitness, the eventual benefits of the relationship, and the importance of the resident bacteria’s properties. This research provides valuable clues on how endosymbioses arise and stabilize over time. Endosymbiosis Mitochondria
Cohabitation and Compromise
When a bacterium gets stuck in an entirely alien host cell, it is in serious trouble. The decades-long survival, reproduction, and therefore congregation to the next germinate are essential for the constitute’s continued existence. To be sure, the host also must protect itself from being overwhelmed by bacteria so that an exploitive relationship is not made where the bacterium kills its host which in turn would die as well. The complex beginnings of an endosymbiotic partnership provided a level playing field for researchers from ETH Zürich, who carried out a fascinating study.
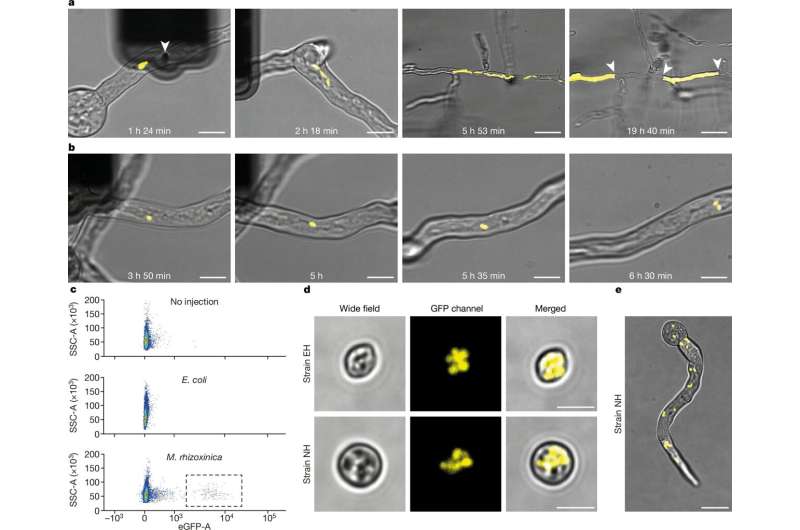
Graduate student Elizabeth Shank combined bacteria — including E. coli and a Mycetohabitans species — with germ tubes (green) emerging from the spores of the fungus Rhizopus microsporus to demonstrate for the first time that a murine host must inject bacteria as part of fungal invasion. This permitted them to look at forced cohabitation beneath a magnifying glass from the get-go. What they discovered, however, was an eye-opener suggesting that these ancient endosymbiotic relationships were highly unstable.
Fitness challenges and adaptations
E. coli bacteria grew alongside the fungus when they were injected, but as the bacteria proliferated the fungus began to encase them in protective walls of immune tissue that stopped them from being carried on into the next generation. Instead, these strains could be transmitted to spores generated by the fungus Mycetohabitans and therefore even to spore-based generations of host ants.
However, it was at the cost of this endosymbiotic relationship. Fungi with the bacteria on board germinated less frequently, and when they did germinate, they grew more slowly than mutant fungi that had jettisoned their partners. “Initially the endosymbiosis reduced the overall fitness of infected fungi,” says lead author Gabriel Giger, a PhD candidate who carried out the experimental work. The researchers then selected fungi with spores bearing bacteria over successive generations, which was a way to force the system to evolve and recover. The fungus had altered in response to its host, and over time the two produced bioactive compounds that could be symbiotically beneficial.
Parenthood: The Road to Endosymbiosis
It also shows how early endosymbiotic associations were a confounding factor on the threshold of adaptations vital for them to survive. If the host’s fitness goes down at first… it may just shorten those systems’ life span in nature,” Giger says. For new endosymbioses to form and persist, it needs to be significantly beneficial to do so.
It is not a given that the hitherto prospective resident bacteria develops properties that facilitate endosymbiosis, and the host has to be prepared to compromise somewhat to incorporate this new organism into its life. “Endosymbioses have shown how ultimately successful they can be in evolution,” she says, referencing mitochondria as an example, which evolved from ancient endosymbiotic bacteria.
The research offers an important window into how some of the most vital elements in life began forming partnerships, and it provides insights into how these complex symbiotic relationships may grow over time.
