Ammonia has emerged as a promising solution for transporting hydrogen, a crucial step in the transition to green energy. The hydrogen economy holds immense potential, but the challenges of liquefying and transporting hydrogen have hindered its widespread adoption. This article delves into the groundbreaking research that has shed new light on the role of iron catalysts in the decomposition of ammonia, paving the way for more efficient hydrogen production and storage.
Unlocking the Secrets of Ammonia Decomposition
Ammonia has long been considered a promising method of transporting hydrogen, a key component in the quest for sustainable energy. However, the efficient conversion of ammonia back into its starting materials – nitrogen and hydrogen – has remained a challenge. That is, until a team of researchers from around the world delved into the intricacies of the iron catalyst, a crucial component in this process.
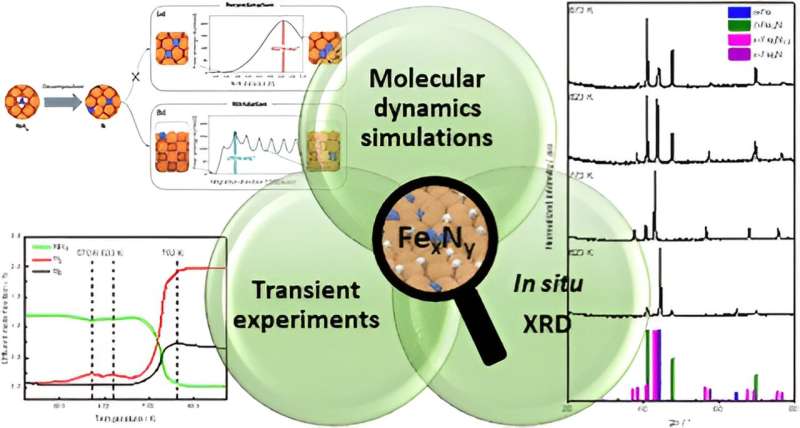
The researchers, led by Professor Martin Muhler from the Ruhr University Bochum and the Max Planck Institute for Chemical Energy Conversion, have made a groundbreaking discovery. By closely examining the behavior of the iron catalyst during ammonia decomposition, they have gained valuable insights into the mechanisms underlying this reaction. This knowledge can be leveraged to develop more efficient catalysts, ultimately paving the way for a more streamlined and cost-effective hydrogen production and transportation system.
Overcoming the Challenges of Hydrogen Storage and Transport
The transition to a hydrogen-based economy has long been a goal for many nations seeking to reduce their carbon footprint and address the pressing issue of climate change. However, the storage and transportation of hydrogen have posed significant challenges. Hydrogen, being the lightest element, is notoriously difficult to liquefy, requiring extremely low temperatures and high pressures.
This is where the conversion of hydrogen into ammonia comes into play. Ammonia, a compound consisting of nitrogen and hydrogen, can be liquefied at much higher temperatures, making it a more practical solution for transportation and storage. The research team’s findings on the iron catalyst used in the decomposition of ammonia could revolutionize this process, unlocking new possibilities for the widespread adoption of hydrogen as a clean energy source.
Pushing the Boundaries of Catalytic Innovation
The research team’s exploration of the iron catalyst has not only yielded valuable insights into ammonia decomposition but has also highlighted the importance of innovative catalytic solutions in the energy sector. Catalysts play a pivotal role in chemical reactions, acting as facilitators that can significantly improve efficiency and reduce the energy required for a process.
In the case of ammonia decomposition, the researchers discovered that conventional iron catalysts often led to the formation of undesirable iron nitrides, hindering the desired reaction. By delving deeper into the complex molecular dynamics of the process, the team was able to identify the mechanisms behind this side reaction and pave the way for the development of more efficient catalysts. This breakthrough has the potential to revolutionize the way we approach hydrogen storage and transportation, ultimately contributing to a more sustainable energy future.
