Scientists have earlier created an approach to make hydrogen from ammonia with the help of a catalyst at lower temperatures than is any other method available by now, and the discovery could prove significant for energy systems in the future
Unlocking the promise of ammonia
High energy density and an easy transition to a hydrogen economy for large-scale fuel cell applications — why hydrogen keeps everyone excited For decades now, we have been told that Hydrogen is the future of energy. The problem is that we do not know an easy way to reliably and inexpensively make a lot of it.
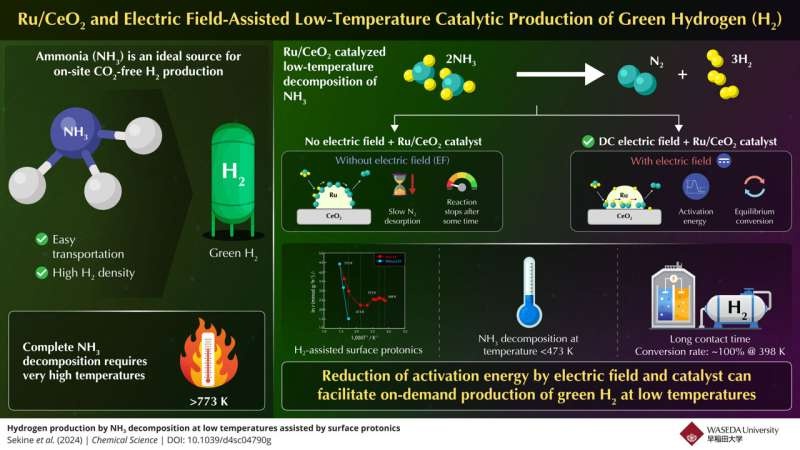
Ammonia, a rising star on the exalted list of hydrogen carriers The fact that ammonia is both widely available, highly hydrogen dense (17.6% by mass) and is easily liquefied as well as transported makes it a candidate fuel of interest compared to others [156–158]. However, the limitation it had is that it needs very high temperatures (>773K) to decompose and give out Hydrogen.
A team from Waseda University has joined forces with Yanmar Holdings to offer a solution. Here demonstrates that a high activity and easily fabricated Ru/CeO2 catalyst can be generated and used to achieve reliable, direct, electrically-driven ammonia-to-hydrogen conversion at very lower temperatures by applying an electric field.
Breakthrough: Electric Field Catalysis
The innovativeness of the team lies in their proposition that one of the rate determining step in ammonia decomposition process is being addressed. The researchers found that low-temperature desorption of nitrogen was the slow step under the cold conditions, while dissociation of N-H bonds was rate limiting under hot plant.
Electric field-assisted catalytic reactions were identified to be key to overcoming these obstacles. They did this by using a DC electric field, which is found to substantially boost proton conduction on the surface of the catalyst and thus it reduces the activation energy at which reaction occur—and hence lowering operating temperatures.
This new approach turned its fortunes around. The team reveals that even at temperatures below 473 K, their proposed system succeeded in greatly enhancing the ammonia decomposition efficiency; for example, with an adequate contact time between the ammonia feed and the catalyst, they were able to convert all of it at only 398 K, on par with a complete conversion rate than an equilibrium state can provide.
Critical to this success is the driving force of the electric field, which can enhance surface protonics (i.e., promote hopping of protons on the catalyst surface). This phenomenon can drop the apparent activation energies of Vo/H transformation and N2 dissociation in ammonia conversion, thereby promoting a rapid low temperature decomposition.
Conclusion
Besides, this new non-precious catalysts for high efficiency low temperature ammonia-to-hydrogen conversion is a major advance in the area of sustainable energy solutions. The use of ammonia along with the organic ions [24] and its benefits [29,30,31] in conjunction with the catalysis effect due to an applied electric field are effectively opening a way for ammonia and “cleaner” low-molecular-weight alkanes alternative fuels. This new system provides a ray of hope for producing carbon-free hydrogen on-demand faster and simpler, thus smoothening the path towards a more eco-friendly and sustainable future.
