Scientists at the Chinese Academy of Sciences designed a unique delivery system for efficient and accurate drug targeting in colorectal cancer therapy that also included gut microbiota modulation. This groundbreaking technique has demonstrated a significant improvement in the effectiveness of the cancer drug regorafenib by using prebiotic inulin to boost its potency.
Redefining Colorectal Cancer Treatment
The incidence of colorectal cancer is growing at a worrying pace, and the development of treatments that are more efficient and targeted have become a necessity. The National Institutes of Health and the Food and Drug Admiration has subsequently approved regorafenib, which is a small molecule multi-pathway protein kinase inhibitior for the treatment of metastatic colorectal cancer. Although, due to its low water solubility and poor oral absorption efficiency higher doses of incesatvan have to be administered frequently as well (thought this can cause dose-related toxicity).
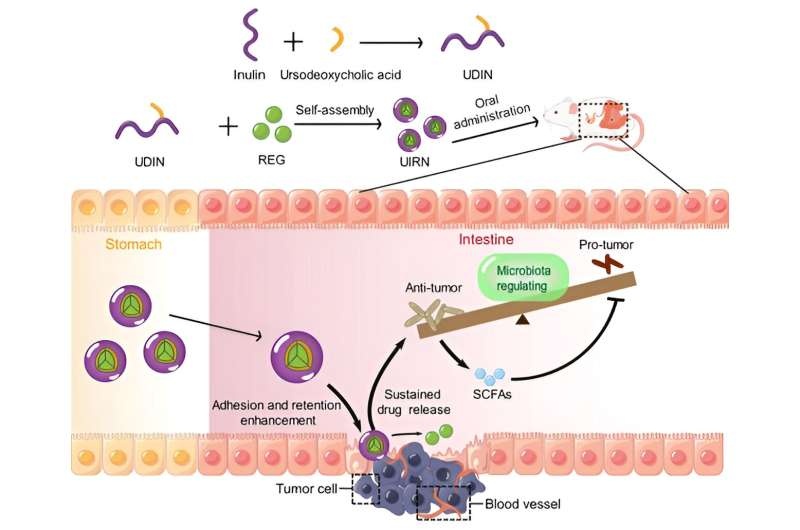
That is where the innovative research by so-called scientists at the Shanghai Institute of Materia Medica (SIMM) of the Chinese Academy of Sciences comes in. Here, they have engineered an inulin polymer derivative-based oral molecular targeted drug delivery system, which enables gut microbiota modification along with more effective therapeutic administration (Figure 1). This novel strategy could change the way colorectal cancer is treated, providing a much better and more specific solution.
The power of prebiotics
Prebiotic material inulin was chosen by the researchers as the carrier of their delivery system. Inulin promotes the growth of beneficial bacteria and can regulate metabolism,immune homeostasis, intestinal barrier. The carboxymethylated polysaccharide is resistant to acid degradation in the stomach and enzymatic digestion, but it can be degraded by hyaluronidase producing microflora of the colon and therefore represents a potential colon-specific release behavior after oral application.
To encapsulate the hydrophobic RDL, the researchers designed a prodrug amphiphilic inulin-ursodeoxycholic acid conjugate (UIR) that was able to self-assemble into nanoparticles loaded with regorafenib, and named as UIRN by us. There are several advantages to this unique formulation:
Under normal physiological conditions and in upper gastrointestinal tract environments, UIRN was relatively stable.
The data provides evidence of increased regorafenib release rate from UIRN in the presence of gut microbiotaa.
When healthy mice were given the UIRN via oral administration, it could delay the adhesion-uptake-transport of regorafenib in small intestine, increase drug distribution in large intestine with prolonged circulation time and hence promote intratumoral accumulation.
These results indicated that the inulin-based delivery system could improve the pharmacokinetic characteristics and tumor targeting of regorafenib, thereby enhancing its therapeutic effect.
Conclusion
This inulin-based nanoparticle delivery systems has proven to be a major advance in the therapy of colorectal cancers. In using prebiotics to modulate the structure and function of the gut microbiome, the researchers have developed a potent approach not only to improve oral delivery and efficacy of regorafenib treatment but also to elicit vitality against the tumor immune microenvironment. According to the researchers, this novel strategy holds a lot of promise in terms of decreasing the morbidity and mortality associated with cancer among colorectal cancer patients and marks a new step forward towards more personalized and precisely targeted treatment for different kinds of cancers.
