Dive into the fascinating world of Artemisia annua, a plant celebrated for its antimalarial properties. Discover how researchers from Shanghai Jiao Tong University unraveled the complex metabolic disruptions in a mutant strain, paving the way for enhanced antimalarial drug production.
The Metabolic Maze of Artemisia
One of the amazing things about Artemisia annua is that it produces artemisinin, one of the most powerful antimalarials. Nevertheless, its metabolic routes were widely investigated in the past mainly for artemisinin biosynthesis, leaving out the rest of the pathways operating therein from knowledge.
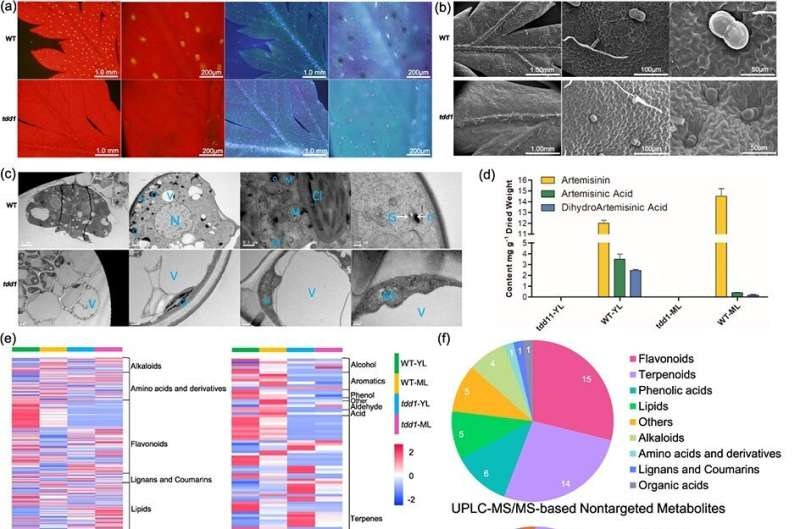
Published in Horticulture Research, scientists from Shanghai Jiao Tong University have identified and reported a detailed characterization of metabolic perturbation in the mutant strain (named as TRICHOME DEVELOPMENTAL DEFECTS 1 -tdd1) of Artemisia annua. This mutant showed a marked reduction in the function of its glandular secretory trichomes (GSTs), necessary for artemisinin biosynthesis.
Using advanced multi-omics profiling, the team made a surprising discovery: Artemisinin and its precursors were essentially absent in both young and mature leaves, suggesting massive metabolic pathway disruption. We showed that instead of being sequestered, heme was readily available for activation by GSTs in the biosynthesis of this key antimalarial molecule [9], highlighting the importance of GST in quinine biosynthesis.
Unraveling the Metabolic Tapestry
This paper shows that these metabolic perturbations extend far beyond effects on artemisinin biosynthesis in the tdd1 mutant. A total of 836 metabolites, consisting mostly flavonoid and terpenoid compounds, were identified based on Liquid Chromatography-Mass Spectrometry (LC–MS) (399 detected metabolites) and Gas Chromatography-Mass Spectrometry (GC–MS) methods (658 detected metabolites), many of which did not express in the mutant strain.
Further study revealed that the Mevalonate Pathway (MVA) and Methylerythritol Phosphate Pathway (MEP) pathways required for secondary metabolite synthesis differed significantly. Consistent with the broader effects of GST defects on metabolite production, the phylogenetic analysis suggests that components of previous proteomics studies have a significant effect on secondary metabolism in higher plants.
The strategy of multi-omics analysis complements each other and places the study within a comprehensive picture that can reveal how complicated metabolic networks in Artemisia annua concerning its glandular secretory trichomes functions.
Conclusion
This work may allow the amelioration of an antimalarial drug, targeting unique metabolic pathways in Artemisia annua. Understanding the genetic and metabolic framework of the plant’s glandular secretory trichomes may guide improvements in cultivation and yield increases through genetic modifications. Further, this work paves an incredibly rich area in Artemisia annua to explore the unusual and more complex secondary metabolites, possibly a gold mine of unknown medicinally valuable compounds apart from artemisinin. In doing so, the researchers have unlocked key Nature Reviews Drug Discovery Metabolic mysteries of this extraordinary plant that will pave the way for an unprecedented generation of new advancements in malaria therapeutics.
