Researchers have made a significant breakthrough in understanding the role of iron catalysts in the decomposition of ammonia, a crucial step in the production of green hydrogen. This discovery could pave the way for more efficient processes to convert hydrogen into a more easily transportable form and back again. Hydrogen economy and ammonia are two key concepts that are explored in this article.
Unlocking the Potential of Ammonia for Hydrogen Transport
The quest for a sustainable and efficient energy solution has led researchers to explore the use of ammonia as a means of transporting hydrogen. Compared to the challenges of liquefying hydrogen at extremely low temperatures, converting it into ammonia offers a more practical alternative.
As Professor Martin Muhler, Head of the Laboratory of Industrial Chemistry in Bochum and Max Planck Fellow at the MPI CEC, explains, “What’s more, the chemical industry already has an established infrastructure for ammonia handling.” This existing infrastructure presents a significant advantage in the transition towards a hydrogen-based economy.
However, the efficient conversion of ammonia back into its starting components of nitrogen and hydrogen remains a crucial challenge. This is where the breakthrough in understanding the role of iron catalysts comes into play.
The Iron Catalyst Unveils its Secrets
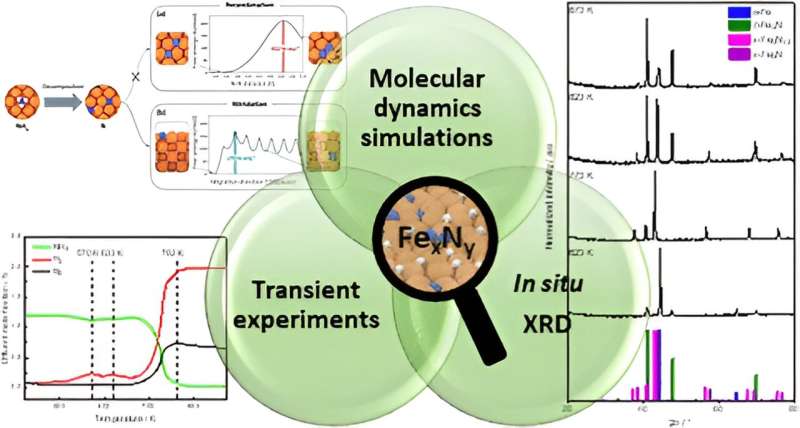
The international research team, comprising scientists from the German Ruhr University Bochum, the Max Planck Institute for Chemical Energy Conversion (MPI CEC) in Mülheim an der Ruhr, Technische Universität Berlin, and the Italian Institute of Technology in Genoa, has delved deep into the inner workings of an iron catalyst that can split ammonia into nitrogen and hydrogen.
The researchers have identified a critical issue with conventional iron catalysts – they tend to facilitate an undesirable reaction, leading to the formation of iron nitride instead of the desired nitrogen. By conducting detailed experiments and utilizing complex molecular dynamics simulations supported by machine learning, the team has now uncovered the precise mechanism behind this side reaction.
This newfound understanding, as Martin Muhler emphasizes, “can be used to develop more efficient catalysts for splitting ammonia in the future.” The ability to control and optimize the catalytic process is a crucial step towards realizing the full potential of ammonia-based hydrogen transportation.
Leveraging a Century of Ammonia Research
The breakthrough in understanding the iron catalyst’s role in ammonia decomposition is not only a significant scientific achievement but also a testament to the longevity and depth of research in this field.
As Martin Muhler points out, “Ammonia synthesis and decomposition has a long track record,” with scientific publications dating back over a century. This includes the groundbreaking work of Muhler’s doctoral supervisor, Gerhard Ertl, who was awarded the Nobel Prize in 2007 for his research in this area.
By building upon this rich history of knowledge and combining it with modern analytical techniques and computational power, the research team has unlocked new insights that can drive further innovation. This collaborative effort, spanning multiple institutions and disciplines, demonstrates the power of interdisciplinary collaboration in solving complex scientific challenges.
