Researchers from the Chinese Academy of Sciences have uncovered a novel approach to improving the conversion of CO₂ to ethylene (C₂H₄) through the strategic manipulation of boron-imidazolate frameworks (BIFs). This breakthrough could hold the key to more efficient and sustainable carbon capture and utilization. Metal-organic frameworks and covalent organic frameworks have long been touted as promising solutions for carbon capture and conversion, and this study sheds light on the intricate interplay between material structure and catalytic performance.
Unlocking the Potential of Boron-Imidazolate Frameworks
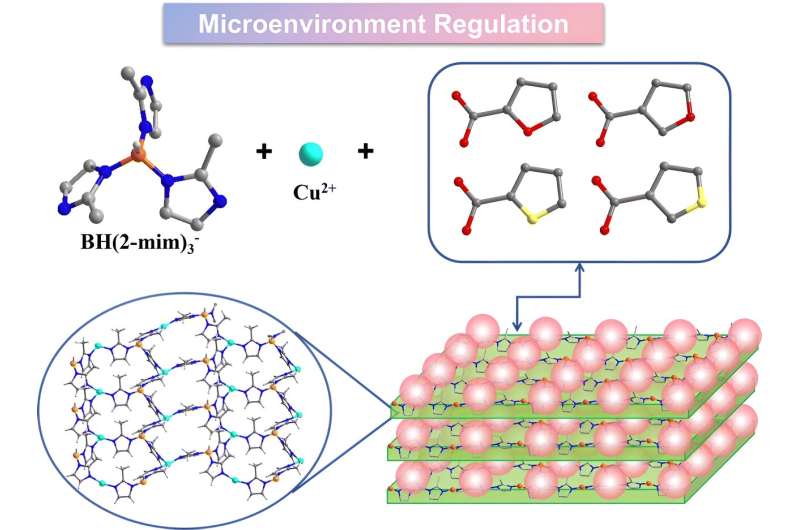
Crystalline boron-imidazolate frameworks (BIFs) possess a unique structure that combines covalent bonds (B–N) and metal coordination bonds (M–N), making them a fascinating hybrid between metal-organic frameworks (MOFs) and covalent organic frameworks (COFs). This novel class of materials has captured the attention of researchers seeking to harness their potential for a wide range of applications, including catalysis, gas storage, and separation.
In this groundbreaking study, the researchers from the Fujian Institute of Research on the Structure of Matter of the Chinese Academy of Sciences have taken a deep dive into the world of BIFs, focusing on their ability to enhance the electrochemical reduction of CO₂ to the valuable product, ethylene (C₂H₄). By carefully manipulating the coordination microenvironment within the BIF structures, the team was able to achieve remarkable improvements in both catalytic activity and selectivity towards C₂H₄ formation.
Unlocking the Secrets of Coordination Microenvironments
The key to the researchers’ success lies in their ability to construct a series of isostructural two-dimensional (2D) BIFs, each with a unique coordination microenvironment. By using a specific ligand, KBH(mim)3, the team was able to create four distinct BIF crystals (BIF-151 to BIF-154) that share the same core framework and metal coordination environment. However, the researchers then introduced different monocarboxylate ligands with varying substituent elements and positions, effectively modifying the surrounding layers and the overall coordination microenvironment.
Through this innovative approach, the researchers demonstrated that the catalytic activity and selectivity towards C₂H₄ production are not solely dependent on the active metal sites, but are also heavily influenced by the coordination microenvironment. The composition and spatial arrangement of the substituent elements within the BIF structure played a crucial role in determining the efficiency and selectivity of the CO₂ electroreduction process.
Implications for Sustainable Carbon Capture and Utilization
The findings of this study hold significant implications for the field of carbon capture and utilization. By unlocking the potential of BIFs and their ability to selectively convert CO₂ into valuable C₂H₄, the researchers have paved the way for more efficient and sustainable carbon recycling strategies. The ability to fine-tune the coordination microenvironment within the BIF structure opens up new avenues for optimizing catalytic performance and targeting specific products, such as the highly sought-after C₂H₄.
This breakthrough showcases the importance of understanding the intricate relationship between material structure and catalytic behavior, and highlights the potential for MOFs and COFs to play a transformative role in addressing the global challenge of climate change. As the world continues to grapple with the pressing need for innovative solutions to reduce greenhouse gas emissions, the insights gained from this study could serve as a valuable foundation for the development of next-generation carbon capture and utilization technologies.
